Exam 6: Thermochemistry
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
What is the standard enthalpy change for the combustion of liquid cyclopentane,C5H10?
2C5H10(l)+ 15O2(g)→ 10CO2(g)+ 10H2O(l)
Substance
ΔH°f (kJ/mol)
C5H10(l)
-105.6
CO2(g)
-393.5
H2O(l)
-285.8
Free
(Multiple Choice)
4.9/5  (30)
(30)
Correct Answer:
E
The overall chemical equation resulting from the sum of the following three steps is
2C(s)+ 2H2O(g)→ 2CO(g)+ 2H2(g)
CO(g)+ H2O(g)→ CO2(g)+ H2(g)
CO(g)+ 3H2(g)→ CH4(g)+ H2O(g)
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
A
Which substance has a standard enthalpy of formation equal to zero at 25°C?
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
C
The reaction of iron with hydrochloric acid is represented by the following thermochemical equation.
Fe(s)+ 2HCl(aq)→ FeCl2(aq)+ H2(g); ΔH° = -87.9 kJ
If,in a particular experiment,1.56 kJ of heat was released at constant pressure,what volume of H2(g),measured at STP,was produced? (R = 0.0821 L • atm/(K • mol))
(Multiple Choice)
4.8/5  (34)
(34)
Calculate ΔU of a gas for a process in which the gas absorbs 29 J of heat and does 31 J of work by expanding.
(Multiple Choice)
4.7/5  (41)
(41)
If q = 60 kJ and w = 96 kJ for a certain process,that process
(Multiple Choice)
4.7/5  (38)
(38)
Which of the following processes will result in the lowest final temperature of the metal-water mixture at equilibrium?
(Multiple Choice)
4.7/5  (33)
(33)
Combustion of 3.65 g of liquid benzene (C6H6)causes a temperature rise of 21.1°C in a constant-pressure calorimeter that has a heat capacity of 7.23 kJ/°C.What is ΔH for the following reaction?
C6H6(l)+ 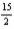 O2(g)→ 6CO2(g)+ 3H2O(l)
O2(g)→ 6CO2(g)+ 3H2O(l)
(Multiple Choice)
4.7/5  (36)
(36)
When 50.0 mL of 1.27 M of HCl(aq)is combined with 50.0 mL of 1.32 M of NaOH(aq)in a coffee-cup calorimeter,the temperature of the solution increases by 8°C.What is the change in enthalpy for this balanced reaction?
HCl(aq)+ NaOH(aq)→ NaCl(aq)+ H2O(l)
Assume that the solution density is 1.00 g/mL and the specific heat capacity of the solution is 4.18 J/g⋅°C.
(Multiple Choice)
4.7/5  (38)
(38)
From the following information,determine ΔH°f of malonic acid,CH2(COOH)2(s).
CH2(COOH)2(s)+ 2O2(g)→ 3CO2(g)+ 2H2O(l); ΔH° = -861.0 kJ
Substance
ΔH°f (kJ/mol)
CO2(g)
-393.5
H2O(l)
-285.8
(Multiple Choice)
4.9/5  (33)
(33)
When 0.0500 mol of HCl(aq)is reacted with 0.0500 mol of NaOH(aq)in 50.0 mL of water,the temperature of the solution increases by 5.99°C.What is the enthalpy of reaction for the following thermochemical equation?
HCl(aq)+ NaOH(aq)→ NaCl(aq)+ H2O(l)
Assume that the heat capacity of the solution and calorimeter is 465.4 J/°C.
(Multiple Choice)
4.8/5  (35)
(35)
The skydiver plunging to earth possesses what type(s)of energy?
(Multiple Choice)
4.9/5  (33)
(33)
A 58.7-g sample of nickel (s = 0.443 J/(g ∙ °C)),initially at 147.4°C,is placed in an insulated vessel containing 184.0 g of water (s = 4.18 J/(g ∙ °C)),initially at 29.9°C.Once equilibrium is reached,what is the final temperature of the metal-water mixture? Neglect the heat capacity of the vessel.
(Multiple Choice)
4.9/5  (35)
(35)
How much heat must be applied to a 40.2-g sample of gold (s = 0.129 J/(g ∙ °C))in order to raise its temperature from 28.2°C to 351.5°C?
(Multiple Choice)
4.8/5  (32)
(32)
Under conditions of constant pressure,for which of the following reactions is the magnitude of pressure-volume work going to be greatest?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following reactions corresponds to the thermochemical equation for the standard enthalpy of formation of N,N-diethyl-m-toluamide,C12H17NO(l),the active ingredient in some insect repellents?
(Multiple Choice)
4.8/5  (31)
(31)
The reaction of iron with hydrochloric acid is represented by the following thermochemical equation.
Fe(s)+ 2HCl(aq)→ FeCl2(aq)+ H2(g); ΔH° = -87.9 kJ
In which of the following experiments would the temperature rise the most?
(Multiple Choice)
5.0/5  (29)
(29)
Showing 1 - 20 of 119
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)