Deck 7: Quantum Theory of the Atom
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/68
Play
Full screen (f)
Deck 7: Quantum Theory of the Atom
1
What is the energy of a photon of electromagnetic radiation with a frequency of 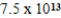 Hz? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)
Hz? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)
A)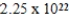 J
J
B)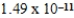 J
J
C)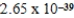 J
J
D)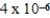 J
J
E)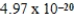 J
J
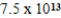 Hz? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)
Hz? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)A)
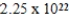 J
JB)
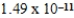 J
JC)
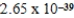 J
JD)
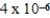 J
JE)
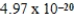 J
J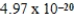 J
J 2
A laser emits photons having an energy of 3.74 × 10-19 J.What color would be expected for the light emitted by this laser? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J ⋅ s)
A)yellow to orange
B)orange to red
C)green
D)violet
E)blue
A)yellow to orange
B)orange to red
C)green
D)violet
E)blue
green
3
Which type of electromagnetic radiation has the lowest frequency?
A)microwaves
B)visible
C)ultraviolet
D)infrared
E)radio waves
A)microwaves
B)visible
C)ultraviolet
D)infrared
E)radio waves
radio waves
4
What is the frequency of a photon having a wavelength of 141.8 nm? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)
A)2.12 × 10-3 Hz
B)2.14 × 1026 Hz
C)2.12 × 1015 Hz
D)1.4 × 10-36 Hz
E)1.4 × 10-18 Hz
A)2.12 × 10-3 Hz
B)2.14 × 1026 Hz
C)2.12 × 1015 Hz
D)1.4 × 10-36 Hz
E)1.4 × 10-18 Hz

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements is incorrect?
A)As the energy of a photon increases,its frequency decreases.
B)As the wavelength of a photon increases,its energy decreases.
C)The product of wavelength and frequency of electromagnetic radiation is a constant.
D)As the wavelength of a photon increases,its frequency decreases.
E)As the frequency of a photon increases,its wavelength decreases.
A)As the energy of a photon increases,its frequency decreases.
B)As the wavelength of a photon increases,its energy decreases.
C)The product of wavelength and frequency of electromagnetic radiation is a constant.
D)As the wavelength of a photon increases,its frequency decreases.
E)As the frequency of a photon increases,its wavelength decreases.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
6
What is the frequency of photons that have molar energy of 339.00 kJ/mol?
(c = 3.00 × 108 m/s,h = 6.63 × 10-34 J·s,NA = 6.02 × 1023 mol-1)
A)5.63×10-19 Hz
B)8.49×1014 Hz
C)1.69×10-10 Hz
D)5.87×10-31 Hz
E)2.83×106 Hz
(c = 3.00 × 108 m/s,h = 6.63 × 10-34 J·s,NA = 6.02 × 1023 mol-1)
A)5.63×10-19 Hz
B)8.49×1014 Hz
C)1.69×10-10 Hz
D)5.87×10-31 Hz
E)2.83×106 Hz

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
7
Rank the following regions of the electromagnetic spectrum in order of decreasing frequency.
X rays,Microwaves,Infrared,Ultraviolet
A)infrared,microwaves,ultraviolet,x rays
B)x rays,microwaves,infrared,ultraviolet
C)microwaves,infrared,ultraviolet,x rays
D)microwaves,ultraviolet,infrared,x rays
E)x rays,ultraviolet,infrared,microwaves
X rays,Microwaves,Infrared,Ultraviolet
A)infrared,microwaves,ultraviolet,x rays
B)x rays,microwaves,infrared,ultraviolet
C)microwaves,infrared,ultraviolet,x rays
D)microwaves,ultraviolet,infrared,x rays
E)x rays,ultraviolet,infrared,microwaves

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
8
What is the energy per mole of photons with a wavelength of 307.1 nm?
(c=3.00×108 m/s,h=6.63×10-34 J·s,NA=6.02×1023mol-1)
A) 6.48×10-19 kJ/mol
B)1.85×1014 kJ/mol
C)5.88×1035 kJ/mol
D)9.77×1014 kJ/mol
E)3.9×102 kJ/mol
(c=3.00×108 m/s,h=6.63×10-34 J·s,NA=6.02×1023mol-1)
A) 6.48×10-19 kJ/mol
B)1.85×1014 kJ/mol
C)5.88×1035 kJ/mol
D)9.77×1014 kJ/mol
E)3.9×102 kJ/mol

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
9
Which type of electromagnetic radiation has the longest wavelength?
A)red light
B)x rays
C)gamma rays
D)microwaves
E)blue light
A)red light
B)x rays
C)gamma rays
D)microwaves
E)blue light

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
10
What is the wavelength of photons that have molar energy of 515 kJ/mol? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s,NA = 6.02 × 1023 mol-1)
A)1.29 × 106 nm
B)8.55 × 10-10 nm
C)233 nm
D)3.86 × 10-22 nm
E)2.33 × 105 nm
A)1.29 × 106 nm
B)8.55 × 10-10 nm
C)233 nm
D)3.86 × 10-22 nm
E)2.33 × 105 nm

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
11
What is the frequency of a photon having an energy of 4.91 × 10-17 ? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)
A)2.22× 1025 Hz
B)4.05× 10-9 Hz
C)7.41× 1016 Hz
D)4.5× 10-26 Hz
E)1.47× 10-8 Hz
A)2.22× 1025 Hz
B)4.05× 10-9 Hz
C)7.41× 1016 Hz
D)4.5× 10-26 Hz
E)1.47× 10-8 Hz

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
12
Which type of electromagnetic radiation has the lowest energy?
A)gamma rays
B)x rays
C)red light
D)blue light
E)radio waves
A)gamma rays
B)x rays
C)red light
D)blue light
E)radio waves

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
13
When a particular metal is illuminated with photons,one electron is observed for each absorbed photon.What effect would decreasing the wavelength and number of photons have on the electrons leaving the surface?
A)There would be more electrons leaving the surface.
B)They would have higher kinetic energy.
C)The electron velocity would be lower.
D)The kinetic energy of the electrons would be lower.
E)Two photons might be required to eject the electrons.
A)There would be more electrons leaving the surface.
B)They would have higher kinetic energy.
C)The electron velocity would be lower.
D)The kinetic energy of the electrons would be lower.
E)Two photons might be required to eject the electrons.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
14
What is the wavelength of a photon having a frequency of 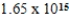 Hz? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)
Hz? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)
A)181 nm
B)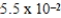 nm
nm
C)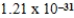 nm
nm
D)0.328 nm
E)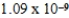 nm
nm
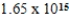 Hz? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)
Hz? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)A)181 nm
B)
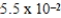 nm
nmC)
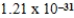 nm
nmD)0.328 nm
E)
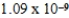 nm
nm
Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
15
What is the wavelength of a photon that has an energy of 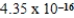 J? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)
J? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)
A)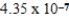 nm
nm
B)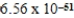 nm
nm
C)0.457 nm
D)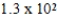 nm
nm
E)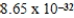 nm
nm
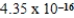 J? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)
J? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)A)
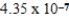 nm
nmB)
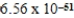 nm
nmC)0.457 nm
D)
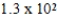 nm
nmE)
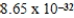 nm
nm
Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
16
A photon of red light has a ____ frequency and a ____ wavelength than a photon of blue light.
A)lower,longer
B)higher,shorter
C)lower,shorter
D)higher,longer
E)lower,lower
A)lower,longer
B)higher,shorter
C)lower,shorter
D)higher,longer
E)lower,lower

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
17
A light emitting diode (L.E.D.)emits photons with an energy of 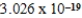 J.What is the energy per mole of photons emitted?
J.What is the energy per mole of photons emitted?
A)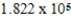 J/mol
J/mol
B)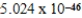 J/mol
J/mol
C)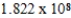 J/mol
J/mol
D)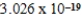 J/mol
J/mol
E)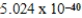 J/mol
J/mol
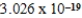 J.What is the energy per mole of photons emitted?
J.What is the energy per mole of photons emitted? A)
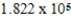 J/mol
J/molB)
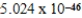 J/mol
J/molC)
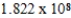 J/mol
J/molD)
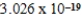 J/mol
J/molE)
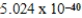 J/mol
J/mol
Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
18
What is the energy of a photon of electromagnetic radiation with a wavelength of 963.5 nm? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)
A)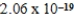 J
J
B)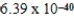 J
J
C)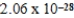 J
J
D)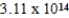 J
J
E)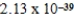 J
J
A)
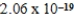 J
JB)
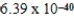 J
JC)
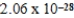 J
JD)
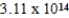 J
JE)
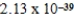 J
J
Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
19
Based on the photoelectric effect,Einstein proposed the idea that
A)the energy of a single particle or photon of light is inversely proportional to its frequency.
B)the wavelength of light is inversely proportional to its frequency.
C)particles can show characteristics of waves under certain experimental conditions.
D)the energy of an object is proportional to its mass.
E)light has particle-like properties.
A)the energy of a single particle or photon of light is inversely proportional to its frequency.
B)the wavelength of light is inversely proportional to its frequency.
C)particles can show characteristics of waves under certain experimental conditions.
D)the energy of an object is proportional to its mass.
E)light has particle-like properties.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
20
What is the wavelength of a photon having a frequency of 49.3 THz? (1 THz = 1015 Hz,c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)
A)0.164 nm
B)3.27 × 10-23 nm
C)9.81× 10-15 nm
D)6.08 nm
E)6.09 × 10-3 nm
A)0.164 nm
B)3.27 × 10-23 nm
C)9.81× 10-15 nm
D)6.08 nm
E)6.09 × 10-3 nm

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
21
If the x-component of the velocity of an electron can be measured only to a precision of 5 × 10-2 m/s,what is the minimum uncertainty of the position of the electron in the x-direction? ( 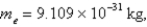
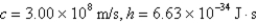
A)9.5 × 1032 m
B)1 × 10-3 m
C)1.1 × 10-33 m
D)8.6 × 102 m
E)2 × 101 m
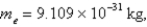
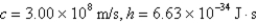
A)9.5 × 1032 m
B)1 × 10-3 m
C)1.1 × 10-33 m
D)8.6 × 102 m
E)2 × 101 m

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
22
From the Bohr model of the hydrogen atom,we can conclude that the energy required to excite an electron from n = 4 to n = 5 is ____ the energy required to excite an electron from n = 3 to 4.
A)less than
B)greater than
C)equal to
D)either equal to or less than
E)either equal to or greater than
A)less than
B)greater than
C)equal to
D)either equal to or less than
E)either equal to or greater than

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
23
What is the wavelength of light emitted when the electron in a hydrogen atom undergoes a transition from level n = 6 to level n = 2?
(c=3.00×108 m/s,h=6.63×10-34 J·s,RH=2.179×10-18 J)
A)4×10-7 m
B)1.61×10-27 m
C)2.43×106 m
D)4.84×10-19 m
E)7.3×1014 m
(c=3.00×108 m/s,h=6.63×10-34 J·s,RH=2.179×10-18 J)
A)4×10-7 m
B)1.61×10-27 m
C)2.43×106 m
D)4.84×10-19 m
E)7.3×1014 m

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
24
What is the frequency of light emitted when the electron in a hydrogen atom undergoes a transition from level n = 6 to level n = 4?
(c=3.00×108 m/s,h=6.63×10-34 J·s,RH=2.179×10-18 J)
A)2.63×10-6 Hz
B)3.8×105 Hz
C)3.97×1027 Hz
D)7.57×10-20 Hz
E)1×1014 Hz
(c=3.00×108 m/s,h=6.63×10-34 J·s,RH=2.179×10-18 J)
A)2.63×10-6 Hz
B)3.8×105 Hz
C)3.97×1027 Hz
D)7.57×10-20 Hz
E)1×1014 Hz

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
25
A radial probability plot for an electron in an atom,like that shown below, 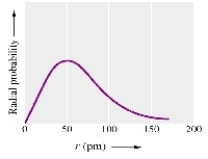
A)specifies the probable speed of the electron at a given radius from the nucleus.
B)specifies the probable momentum of the electron at a given radius from the nucleus.
C)describes the probable energy of the electron at a given radius from the nucleus.
D)gives the probability of finding one electron near another at a given radius from the nucleus.
E)gives the probability of finding the electron at a given radius from the nucleus.

A)specifies the probable speed of the electron at a given radius from the nucleus.
B)specifies the probable momentum of the electron at a given radius from the nucleus.
C)describes the probable energy of the electron at a given radius from the nucleus.
D)gives the probability of finding one electron near another at a given radius from the nucleus.
E)gives the probability of finding the electron at a given radius from the nucleus.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following statements is a valid conclusion from the Heisenberg uncertainty principle?
A)The square of the wave function is proportional to the probability of finding a particle in space.
B)Particles can exhibit wavelike behavior.
C)The orbits proposed by Bohr's model of the atom are correct.
D)An electron in a 2p orbital is always closer to the nucleus than an electron in a 3p orbital.
E)The act of measuring a particle's position changes its momentum,and vice versa.
A)The square of the wave function is proportional to the probability of finding a particle in space.
B)Particles can exhibit wavelike behavior.
C)The orbits proposed by Bohr's model of the atom are correct.
D)An electron in a 2p orbital is always closer to the nucleus than an electron in a 3p orbital.
E)The act of measuring a particle's position changes its momentum,and vice versa.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
27
What is the wavelength of an electron traveling at of 3.74% the speed of light?
(me=9.109×10-31 kg,c=3.00×108 m/s,h=6.63×10-34 J·s)
A)1.54×1010 m
B)2.43×10-12 m
C)6.49×10-11 m
D)1.54×1012 m
E)6.49×10-13 m
(me=9.109×10-31 kg,c=3.00×108 m/s,h=6.63×10-34 J·s)
A)1.54×1010 m
B)2.43×10-12 m
C)6.49×10-11 m
D)1.54×1012 m
E)6.49×10-13 m

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
28
When an electron in an atom makes a transition from n = 4 to n = 5,which of the following statements is/are correct?
I.Energy is emitted.
II.Energy is absorbed.
III.The electron loses energy.
IV.The electron gains energy.
V.The electron cannot make this transition.
A)II and IV
B)II and III
C)I and IV
D)I and III
E)V
I.Energy is emitted.
II.Energy is absorbed.
III.The electron loses energy.
IV.The electron gains energy.
V.The electron cannot make this transition.
A)II and IV
B)II and III
C)I and IV
D)I and III
E)V

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
29
Consider the following energy-level diagram for a particular electron in an atom. 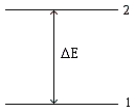 Based on this diagram,which of the following statements is incorrect?
Based on this diagram,which of the following statements is incorrect?
A)The wavelength of a photon emitted by the electron jumping from level 2 to level 1 is given by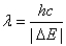 .
.
B)If the electron is in level 1,it may jump to level 2 by absorbing a photon with energy of ΔE.
C)If the electron is in level 1,it may jump to level 2 by absorbing any photon having energy of at least ΔE.
D)We would observe an electron jumping from level 2 to level 1 as a single line in a line spectrum.
E)If the electron is in level 2,it may jump to level 1 by emitting a photon with energy of |ΔE|.
 Based on this diagram,which of the following statements is incorrect?
Based on this diagram,which of the following statements is incorrect?A)The wavelength of a photon emitted by the electron jumping from level 2 to level 1 is given by
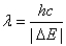 .
.B)If the electron is in level 1,it may jump to level 2 by absorbing a photon with energy of ΔE.
C)If the electron is in level 1,it may jump to level 2 by absorbing any photon having energy of at least ΔE.
D)We would observe an electron jumping from level 2 to level 1 as a single line in a line spectrum.
E)If the electron is in level 2,it may jump to level 1 by emitting a photon with energy of |ΔE|.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
30
Whose postulates account for the line spectrum of an atom?
A)Thomson
B)de Broglie
C)Heisenberg
D)Rutherford
E)Bohr
A)Thomson
B)de Broglie
C)Heisenberg
D)Rutherford
E)Bohr

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following scientists first postulated that the sharp lines in the emission spectra of elements were caused by electrons going from high-energy levels to low-energy levels?
A)Rutherford
B)Pauli
C)Hund
D)de Broglie
E)Bohr
A)Rutherford
B)Pauli
C)Hund
D)de Broglie
E)Bohr

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
32
Who postulated that energy is radiated only when an electron falls from a higher-energy level to a lower-energy level?
A)Bohr
B)Heisenberg
C)Rutherford
D)Einstein
E)Millikan
A)Bohr
B)Heisenberg
C)Rutherford
D)Einstein
E)Millikan

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following statements is incorrect concerning the wave function?
A)The wave function of a particle is a solution to the Schrödinger equation.
B)For an electron in an atom,the square of the wave function decreases rapidly as the distance from the nucleus increases.
C)The square of the wave function is proportional to the probability of finding the particle in a region of space.
D)The value of the wave function gives the location of the particle.
E)The wave function for an electron in an atom is called an atomic orbital.
A)The wave function of a particle is a solution to the Schrödinger equation.
B)For an electron in an atom,the square of the wave function decreases rapidly as the distance from the nucleus increases.
C)The square of the wave function is proportional to the probability of finding the particle in a region of space.
D)The value of the wave function gives the location of the particle.
E)The wave function for an electron in an atom is called an atomic orbital.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
34
The square of the wave function,ψ2,of an electron in an atom
A)is inversely proportional to the distance between the electron and the nucleus.
B)specifies the momentum of the electron.
C)describes the energy of the electron.
D)is proportional to the velocity of the electron.
E)gives the probability of finding the electron in a region of space.
A)is inversely proportional to the distance between the electron and the nucleus.
B)specifies the momentum of the electron.
C)describes the energy of the electron.
D)is proportional to the velocity of the electron.
E)gives the probability of finding the electron in a region of space.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
35
What is the energy per mole of photons having a frequency of 8.44 ×1014Hz?
(c=3.00×108 m/s,h=6.63×10-34 J·s,NA=6.02×1023 mol-1).
A)2.36×10-31 kJ/mol
B)3.37×102 kJ/mol
C)2.14×1023 kJ/mol
D)1.42×10-10 kJ/mol
E)4×102 kJ/mol
(c=3.00×108 m/s,h=6.63×10-34 J·s,NA=6.02×1023 mol-1).
A)2.36×10-31 kJ/mol
B)3.37×102 kJ/mol
C)2.14×1023 kJ/mol
D)1.42×10-10 kJ/mol
E)4×102 kJ/mol

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
36
The contribution for which de Broglie is best remembered in modern science is
A)his statement that no electron can have identical values for all four quantum numbers.
B)his proposal that particles of matter should be associated with wavelike behavior.
C)his statement that an electron can exist in an atom only in discrete energy levels.
D)his statement that elements show periodic repetition of properties.
E)his statement that electrons occupy all the orbitals of a given sublevel singly before pairing begins.
A)his statement that no electron can have identical values for all four quantum numbers.
B)his proposal that particles of matter should be associated with wavelike behavior.
C)his statement that an electron can exist in an atom only in discrete energy levels.
D)his statement that elements show periodic repetition of properties.
E)his statement that electrons occupy all the orbitals of a given sublevel singly before pairing begins.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
37
In Bohr's atomic theory,when an electron moves from one energy level to another energy level more distant from the nucleus,
A)energy is absorbed.
B)light is emitted.
C)energy is emitted.
D)no change in energy occurs.
E)none of these
A)energy is absorbed.
B)light is emitted.
C)energy is emitted.
D)no change in energy occurs.
E)none of these

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
38
What is the wavelength of a 142-g baseball traveling at 90.7 mph? ( 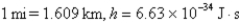 )
)
A)3.13 × 1037 m
B)1 × 10-34 m
C)1.15 × 10-37 m
D)8.68 × 1033m
E)3.2 × 10-38m
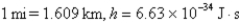 )
)A)3.13 × 1037 m
B)1 × 10-34 m
C)1.15 × 10-37 m
D)8.68 × 1033m
E)3.2 × 10-38m

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
39
The electron in a hydrogen atom,originally in level n = 8,undergoes a transition to a lower level by emitting a photon of wavelength 3745 nm.What is the final level of the electron?
(c=3.00×108 m/s,h=6.63×10-34 J·s,RH=2.179×10-18 J)
A)5
B)6
C)8
D)9
E)1
(c=3.00×108 m/s,h=6.63×10-34 J·s,RH=2.179×10-18 J)
A)5
B)6
C)8
D)9
E)1

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
40
If the location of a particular electron can be measured only to a precision of 0.069 nm,what is the minimum uncertainty in the electron's velocity?
(me=9.109×10-31 kg,c=3.00×108 m/s,h=6.63×10-34 J·s)
A)8.4×105 m/s
B)2.2×105 m/s
C)8.4×10-4 m/s
D)1×10-6 m/s
E)1.7×10-13 m/s
(me=9.109×10-31 kg,c=3.00×108 m/s,h=6.63×10-34 J·s)
A)8.4×105 m/s
B)2.2×105 m/s
C)8.4×10-4 m/s
D)1×10-6 m/s
E)1.7×10-13 m/s

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
41
What is the value of the principal quantum number for an electron in a 5d orbital?
A)-5
B)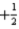
C)5
D)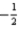
E)2
A)-5
B)
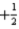
C)5
D)
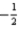
E)2

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
42
How many values are there for the magnetic quantum number when the value of the angular momentum quantum number is 3?
A)12
B)7
C)1
D)4
E)15
A)12
B)7
C)1
D)4
E)15

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
43
An orbital with the quantum numbers n = 5,l = 2,ml = -1 may be found in which subshell?
A)5f
B)5d
C)5p
D)5g
E)5s
A)5f
B)5d
C)5p
D)5g
E)5s

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following statements is incorrect?
A)The set of quantum numbers n = 3,l = 2,ml = 0,ms =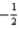 is not permitted because ml = 0.
is not permitted because ml = 0.
B)The set of quantum numbers n = 2,l = 2,ml = 1,ms =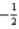 is not permitted because n = l.
is not permitted because n = l.
C)The set of quantum numbers n = 3,l = 2,ml = 1,ms =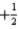 is permitted.
is permitted.
D)The set of quantum numbers n = 3,l = 2,ml = 3,ms =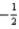 is not permitted because ml exceeds l.
is not permitted because ml exceeds l.
E)The set of quantum numbers n = 4,l = 3,ml = -1,ms = 0 is not permitted because ms = 0.
A)The set of quantum numbers n = 3,l = 2,ml = 0,ms =
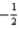 is not permitted because ml = 0.
is not permitted because ml = 0.B)The set of quantum numbers n = 2,l = 2,ml = 1,ms =
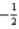 is not permitted because n = l.
is not permitted because n = l.C)The set of quantum numbers n = 3,l = 2,ml = 1,ms =
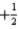 is permitted.
is permitted.D)The set of quantum numbers n = 3,l = 2,ml = 3,ms =
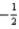 is not permitted because ml exceeds l.
is not permitted because ml exceeds l.E)The set of quantum numbers n = 4,l = 3,ml = -1,ms = 0 is not permitted because ms = 0.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
45
What is the value of the spin quantum number for an electron in a 4d orbital?
A)4
B)2
C)either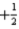 or
or 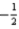
D)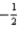
E)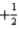
A)4
B)2
C)either
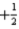 or
or 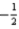
D)
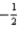
E)
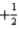

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
46
All the following statements about the quantum numbers are true except
A)ml has 2l + 1 possible values.
B)n may take integral values from 1 to ∞.
C)ml may take integral values of +l to -l,including zero.
D)l may take integral values from 1 to n - 1.
E)ms may take only the values of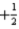 and
and 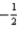 .
.
A)ml has 2l + 1 possible values.
B)n may take integral values from 1 to ∞.
C)ml may take integral values of +l to -l,including zero.
D)l may take integral values from 1 to n - 1.
E)ms may take only the values of
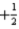 and
and 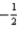 .
.
Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
47
The number of orbitals having a given value of l is equal to
A)2n + 1.
B)2l + 1.
C)n + ml.
D)2ml + 1.
E)l + ml.
A)2n + 1.
B)2l + 1.
C)n + ml.
D)2ml + 1.
E)l + ml.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
48
Which quantum number distinguishes the different shapes of the orbitals?
A)n
B)ml
C)l
D)ms
E)any of these
A)n
B)ml
C)l
D)ms
E)any of these

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following sets of quantum numbers (n,l,ml, ms)is not permissible?
A)2 2 1 +
B)3 1 0 -
C)2 0 0 +
D)2 1 0 +
E)4 0 0 -
A)2 2 1 +

B)3 1 0 -

C)2 0 0 +

D)2 1 0 +

E)4 0 0 -


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
50
The number of orbitals in a p subshell is
A)3.
B)1.
C)7.
D)2.
E)5.
A)3.
B)1.
C)7.
D)2.
E)5.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
51
What is the value of the angular momentum quantum number for an electron in a 1s orbital?
A)2
B)4
C)3
D)0
E)1
A)2
B)4
C)3
D)0
E)1

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following statements is incorrect?
A)The n = 3 shell has three p orbitals.
B)Every p subshell has three orbital.
C)The n = 4 shell has seven f orbitals.
D)An s orbital has a spherical shape.
E)The n = 2 shell has five d orbitals.
A)The n = 3 shell has three p orbitals.
B)Every p subshell has three orbital.
C)The n = 4 shell has seven f orbitals.
D)An s orbital has a spherical shape.
E)The n = 2 shell has five d orbitals.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
53
A possible value of the magnetic quantum number ml for a 5p electron is
A)1.
B)4.
C)-5.
D)6.
E)-3.
A)1.
B)4.
C)-5.
D)6.
E)-3.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following combinations of quantum numbers is permissible?
A)n = 1,l = 2,ml = 0,ms =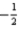
B)n = 3,l = 2,ml = 1,ms =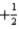
C)n = 3,l = 3,ml = 1,ms =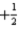
D)n = 2,l = 1,ml = -1,ms = 0
E)n = 4,l = 3,ml = 4,ms =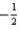
A)n = 1,l = 2,ml = 0,ms =
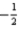
B)n = 3,l = 2,ml = 1,ms =
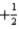
C)n = 3,l = 3,ml = 1,ms =
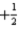
D)n = 2,l = 1,ml = -1,ms = 0
E)n = 4,l = 3,ml = 4,ms =
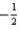

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following subshells does not exist?
A)6g
B)3f
C)3p
D)2s
E)4d
A)6g
B)3f
C)3p
D)2s
E)4d

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
56
Which orbital or orbitals is/are specified by the set of quantum numbers n= 2 and l= 1?
A)2p
B)3s
C)4d
D)1f
E)2d
A)2p
B)3s
C)4d
D)1f
E)2d

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
57
How many p orbitals are in the n = 3 shell?
A)1
B)6
C)0
D)5
E)3
A)1
B)6
C)0
D)5
E)3

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
58
How many orbitals have the set of quantum numbers n =4 and l = 2?
A)5
B)9
C)7
D)1
E)3
A)5
B)9
C)7
D)1
E)3

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following sets of quantum numbers (n,l,ml, ms)refers to a 3d orbital?
A)2 0 0 -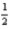
B)5 4 1 -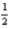
C)4 2 -2 +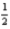
D)4 3 1 -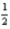
E)3 2 1 -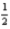
A)2 0 0 -
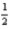
B)5 4 1 -
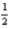
C)4 2 -2 +
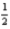
D)4 3 1 -
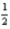
E)3 2 1 -
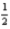

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
60
The angular momentum quantum number is best associated with the
A)shape of the orbital.
B)number of orbitals in a subshell.
C)energy of the orbital.
D)orientation in space of an orbital.
E)none of the above
A)shape of the orbital.
B)number of orbitals in a subshell.
C)energy of the orbital.
D)orientation in space of an orbital.
E)none of the above

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
61
Which hydrogen atom orbital has an energy essentially identical to a 3d orbital?
A)5d
B)4d
C)2s
D)1s
E)3p
A)5d
B)4d
C)2s
D)1s
E)3p

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
62
The _____ of a wave is the number of wavelengths of that wave that pass a fixed point in one unit of time and its unit is _____.
A)frequency,hertz
B)wavelength,meter
C)intensity,candela
D)current,ampere
E)period,second
A)frequency,hertz
B)wavelength,meter
C)intensity,candela
D)current,ampere
E)period,second

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
63
What is the total number of subshells found in the n = 6 shell?
A)6
B)36
C)5
D)7
E)8
A)6
B)36
C)5
D)7
E)8

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following is a representation of a 1s orbital?
A)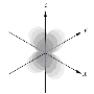
B)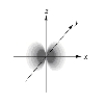
C)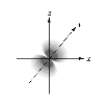
D)
E)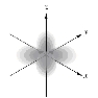
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
65
The wavelength of a gamma ray is 4 × 10-11 m.Calculate the frequency of the ray.
A)32 × 1017/s
B)75 × 1017/s
C)5 × 1019/s
D)12 × 1017/s
E)28 × 1019/s
A)32 × 1017/s
B)75 × 1017/s
C)5 × 1019/s
D)12 × 1017/s
E)28 × 1019/s

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following is a representation of a 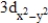 orbital?
orbital?
A)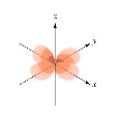
B)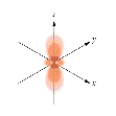
C)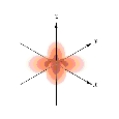
D)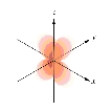
E)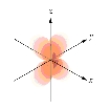
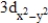 orbital?
orbital?A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
67
The branch of physics that mathematically describes the wave properties of submicroscopic particles is called _____.
A)statistical mechanics
B)quantum mechanics
C)thermodynamics
D)astrodynamics
E)electromagnetism
A)statistical mechanics
B)quantum mechanics
C)thermodynamics
D)astrodynamics
E)electromagnetism

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
68
The frequency of a radio emission is 7.22 × 1013/s.Determine the wavelength of the radio wave.
A)4155 nm
B)3096 nm
C)587 nm
D)828 nm
E)1256 nm
A)4155 nm
B)3096 nm
C)587 nm
D)828 nm
E)1256 nm

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck


