Exam 7: Quantum Theory of the Atom
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
Based on the photoelectric effect,Einstein proposed the idea that
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
E
Which of the following statements is incorrect?
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
A
All the following statements about the quantum numbers are true except
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
D
Rank the following regions of the electromagnetic spectrum in order of decreasing frequency.
X rays,Microwaves,Infrared,Ultraviolet
(Multiple Choice)
4.8/5  (36)
(36)
From the Bohr model of the hydrogen atom,we can conclude that the energy required to excite an electron from n = 4 to n = 5 is ____ the energy required to excite an electron from n = 3 to 4.
(Multiple Choice)
4.9/5  (40)
(40)
What is the frequency of light emitted when the electron in a hydrogen atom undergoes a transition from level n = 6 to level n = 4?
(c=3.00×108 m/s,h=6.63×10-34 J·s,RH=2.179×10-18 J)
(Multiple Choice)
4.8/5  (26)
(26)
Who postulated that energy is radiated only when an electron falls from a higher-energy level to a lower-energy level?
(Multiple Choice)
4.8/5  (33)
(33)
What is the wavelength of an electron traveling at of 3.74% the speed of light?
(me=9.109×10-31 kg,c=3.00×108 m/s,h=6.63×10-34 J·s)
(Multiple Choice)
4.9/5  (32)
(32)
The _____ of a wave is the number of wavelengths of that wave that pass a fixed point in one unit of time and its unit is _____.
(Multiple Choice)
4.8/5  (39)
(39)
What is the energy of a photon of electromagnetic radiation with a wavelength of 963.5 nm? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)
(Multiple Choice)
4.9/5  (27)
(27)
A photon of red light has a ____ frequency and a ____ wavelength than a photon of blue light.
(Multiple Choice)
4.8/5  (25)
(25)
What is the value of the principal quantum number for an electron in a 5d orbital?
(Multiple Choice)
4.8/5  (49)
(49)
A light emitting diode (L.E.D.)emits photons with an energy of 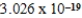 J.What is the energy per mole of photons emitted?
J.What is the energy per mole of photons emitted?
(Multiple Choice)
4.7/5  (40)
(40)
Which of the following is a representation of a 1s orbital?
(Multiple Choice)
4.9/5  (33)
(33)
What is the energy per mole of photons having a frequency of 8.44 ×1014Hz?
(c=3.00×108 m/s,h=6.63×10-34 J·s,NA=6.02×1023 mol-1).
(Multiple Choice)
4.9/5  (36)
(36)
An orbital with the quantum numbers n = 5,l = 2,ml = -1 may be found in which subshell?
(Multiple Choice)
4.8/5  (30)
(30)
Which orbital or orbitals is/are specified by the set of quantum numbers n= 2 and l= 1?
(Multiple Choice)
4.9/5  (27)
(27)
Which type of electromagnetic radiation has the longest wavelength?
(Multiple Choice)
4.7/5  (27)
(27)
Showing 1 - 20 of 68
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)