Deck 10: Molecular Geometry and Chemical Bonding Theory
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/101
Play
Full screen (f)
Deck 10: Molecular Geometry and Chemical Bonding Theory
1
The approximate H-C-C bond angle in ethane,C2H6,is
A)60°.
B)180°.
C)120°.
D)109°.
E)90°.
A)60°.
B)180°.
C)120°.
D)109°.
E)90°.
109°.
2
Which molecule or ion has a trigonal planar molecular geometry?
A)PCl3
B)HCN
C)CO32-
D)HCCH
E)AsF3
A)PCl3
B)HCN
C)CO32-
D)HCCH
E)AsF3
CO32-
3
Which molecule or ion has a trigonal pyramidal molecular geometry?
A)BF3
B)C2H4
C)SO3
D)SO32-
E)CO32-
A)BF3
B)C2H4
C)SO3
D)SO32-
E)CO32-
SO32-
4
Which molecule or ion does not have a trigonal pyramidal molecular geometry?
A)PO33-
B)SO32-
C)NI3
D)BF3
E)XeO3
A)PO33-
B)SO32-
C)NI3
D)BF3
E)XeO3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
5
Which molecule or ion is nonlinear?
A)NO2+
B)SO2
C)NNO
D)CS2
E)SCN-
A)NO2+
B)SO2
C)NNO
D)CS2
E)SCN-

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
6
Which molecule or ion is nonlinear?
A)N2O
B)O3
C)OCN-
D)NO2+
E)CS2
A)N2O
B)O3
C)OCN-
D)NO2+
E)CS2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
7
What is the electron geometry (or electron arrangement)around an atom in a molecule or ion which is surrounded by three lone pairs of electrons and two single bonds.
A)trigonal bipyramidal
B)see-saw or distorted tetrahedron
C)T-shaped
D)linear
E)trigonal planar
A)trigonal bipyramidal
B)see-saw or distorted tetrahedron
C)T-shaped
D)linear
E)trigonal planar

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
8
What is the electron geometry (or electron arrangement)around an atom in a molecule or ion which is surrounded by three lone pairs of electrons and one single bond.
A)tetrahedral
B)trigonal pyramidal
C)trigonal planar
D)bent
E)linear
A)tetrahedral
B)trigonal pyramidal
C)trigonal planar
D)bent
E)linear

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
9
The approximate CCO angle in acetone, 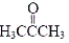 ,is
,is
A)180°.
B)90°.
C)109°.
D)60°.
E)120°.
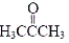 ,is
,isA)180°.
B)90°.
C)109°.
D)60°.
E)120°.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
10
Which molecule or ion is not planar?
A)XeF4
B)NO3-
C)BCl3
D)F2CCF2
E)CF4
A)XeF4
B)NO3-
C)BCl3
D)F2CCF2
E)CF4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
11
Which molecule or ion has a trigonal pyramidal molecular geometry?
A)H2CO
B)H2CCO
C)CH3+
D)CH3-
E)C2H4
A)H2CO
B)H2CCO
C)CH3+
D)CH3-
E)C2H4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
12
What is the molecular geometry around an atom in a molecule or ion which is surrounded by five single bonds and no lone pairs of electrons.
A)trigonal bipyramidal
B)octahedral
C)linear
D)trigonal planar
E)tetrahedral
A)trigonal bipyramidal
B)octahedral
C)linear
D)trigonal planar
E)tetrahedral

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
13
Which molecule or ion is not planar?
A)H2CO
B)NO2-
C)C2F4
D)H2CCO
E)PO43-
A)H2CO
B)NO2-
C)C2F4
D)H2CCO
E)PO43-

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
14
What is the molecular geometry around an atom in a molecule or ion which is surrounded by one lone pair of electrons and four single bonds.
A)see-saw or distorted tetrahedron
B)trigonal bipyramidal
C)linear
D)T-shaped
E)trigonal planar
A)see-saw or distorted tetrahedron
B)trigonal bipyramidal
C)linear
D)T-shaped
E)trigonal planar

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
15
Which molecule or ion is nonlinear?
A)CO2
B)NF2-
C)OCN-
D)NO2+
E)HCCH
A)CO2
B)NF2-
C)OCN-
D)NO2+
E)HCCH

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
16
The approximate H-C-H bond angle in CH3+ is
A)60°.
B)90°.
C)120°.
D)109°.
E)180°.
A)60°.
B)90°.
C)120°.
D)109°.
E)180°.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
17
What is the molecular geometry around an atom in a molecule or ion which is surrounded by two lone pairs of electrons and four single bonds.
A)square planar
B)square pyramidal
C)octahedral
D)bent
E)linear
A)square planar
B)square pyramidal
C)octahedral
D)bent
E)linear

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
18
What is the molecular geometry around an atom in a molecule or ion which is surrounded by one lone pair of electrons and three single bonds.
A)trigonal pyramidal
B)tetrahedral
C)linear
D)bent
E)trigonal planar
A)trigonal pyramidal
B)tetrahedral
C)linear
D)bent
E)trigonal planar

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
19
What is the electron geometry (or electron arrangement)around an atom in a molecule or ion which is surrounded by zero lone pairs of electrons and six single bonds.
A)octahedral
B)square pyramidal
C)square planar
D)bent
E)linear
A)octahedral
B)square pyramidal
C)square planar
D)bent
E)linear

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
20
Which molecule or ion has the same molecular geometry for its central atom as the carbonate ion?
A)H2CO
B)AsCl3
C)PF3
D)CH3-
E)BrO3-
A)H2CO
B)AsCl3
C)PF3
D)CH3-
E)BrO3-

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
21
Which molecule does not have a planar molecular geometry?
A)SO3
B)HCCH
C)N2H4
D)HNNH
E)C2F4
A)SO3
B)HCCH
C)N2H4
D)HNNH
E)C2F4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
22
Which molecule or ion does not have a trigonal pyramidal molecular geometry?
A)AsF3
B)NF3
C)PF3
D)BF3
E)IO3-
A)AsF3
B)NF3
C)PF3
D)BF3
E)IO3-

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
23
What is the molecular geometry of the thiosulfate ion,S2O32-?
A)tetrahedral
B)trigonal bipyramidal
C)pyramidal
D)bent
E)square planar
A)tetrahedral
B)trigonal bipyramidal
C)pyramidal
D)bent
E)square planar

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
24
Which molecule or ion does not have a planar molecular geometry?
A)NO3-
B)BF3
C)F2CO
D)C2H4
E)SO32-
A)NO3-
B)BF3
C)F2CO
D)C2H4
E)SO32-

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
25
The molecular geometry of the CH3- ion is
A)tetrahedral.
B)square planar.
C)square pyramidal.
D)trigonal planar.
E)trigonal pyramidal.
A)tetrahedral.
B)square planar.
C)square pyramidal.
D)trigonal planar.
E)trigonal pyramidal.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
26
Which molecule or ion has the same molecular geometry as SeO32-?
A)SeO3
B)CO32-
C)NO3-
D)SO32-
E)SO3
A)SeO3
B)CO32-
C)NO3-
D)SO32-
E)SO3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
27
The molecular geometry of the CH3+ ion is best described as
A)trigonal planar.
B)pyramidal.
C)linear.
D)bent.
E)tetrahedral.
A)trigonal planar.
B)pyramidal.
C)linear.
D)bent.
E)tetrahedral.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
28
Which molecule or ion has the same molecular geometry for its central atom(s)as BF3?
A)CF4
B)CH3-
C)BF4-
D)C2F4
E)C2F6
A)CF4
B)CH3-
C)BF4-
D)C2F4
E)C2F6

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
29
The molecular geometry of the nitrite ion,NO2- , is best described as
A)pyramidal.
B)trigonal pyramidal.
C)bent.
D)tetrahedral.
E)trigonal planar.
A)pyramidal.
B)trigonal pyramidal.
C)bent.
D)tetrahedral.
E)trigonal planar.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
30
What is the predicted H-N-H bond angle in the ammonium ion?
A)109.5°
B)90°
C)180°
D)120°
E)45°
A)109.5°
B)90°
C)180°
D)120°
E)45°

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
31
The molecular geometry of the ammonium ion,NH4+,is most similar to the molecular geometry of
A)NH3.
B)CH4.
C)N2H4.
D)NH2-.
E)CH3+.
A)NH3.
B)CH4.
C)N2H4.
D)NH2-.
E)CH3+.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
32
The molecule AX3,in which A is the central atom,is polar and obeys the octet rule; therefore,
A)A has two lone pairs.
B)A has one lone pair.
C)A has no lone pairs.
D)A has four bonding pairs.
E)A has three lone pairs.
A)A has two lone pairs.
B)A has one lone pair.
C)A has no lone pairs.
D)A has four bonding pairs.
E)A has three lone pairs.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
33
In phosgene,COCl2,the electron groups are located about the central carbon atom in a ______________ arrangement.
A)square planar
B)trigonal bipyramidal
C)pyramidal
D)trigonal planar
E)tetrahedral
A)square planar
B)trigonal bipyramidal
C)pyramidal
D)trigonal planar
E)tetrahedral

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
34
What is the bond angle in a trigonal planar molecule or ion?
A)109°
B)180°
C)90°
D)72°
E)120°
A)109°
B)180°
C)90°
D)72°
E)120°

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
35
Which molecule or ion is not planar?
A)CO32-
B)Cl2CCCl2
C)HNNH
D)H3O+
E)F2CO
A)CO32-
B)Cl2CCCl2
C)HNNH
D)H3O+
E)F2CO

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
36
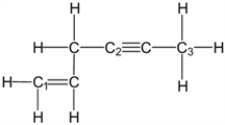
What is the molecular geometry around carbon atom C1?
A)tetrahedral
B)trigonal planar
C)linear
D)trigonal pyramidal
E)bent

A)tetrahedral
B)trigonal planar
C)linear
D)trigonal pyramidal
E)bent

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
37
What is the C-C-H bond angle in H2CCO?
A)109°
B)180°
C)120°
D)144°
E)90°
A)109°
B)180°
C)120°
D)144°
E)90°

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
38
For which of the following molecules or ions do the electron pairs on the central nitrogen atom have a tetrahedral arrangement?
A)FNO
B)NF2-
C)N2F2
D)NO2-
E)NO-
A)FNO
B)NF2-
C)N2F2
D)NO2-
E)NO-

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
39
What is the O-N-O bond angle in the nitrite ion?
A)90°
B)180° and 90°
C)180°
D)120°
E)109°
A)90°
B)180° and 90°
C)180°
D)120°
E)109°

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
40
In the hydronium ion,H3O+,the electron groups are arranged about the central oxygen atom in a
A)tetrahedron.
B)square plane.
C)pyramid.
D)trigonal plane.
E)bent structure.
A)tetrahedron.
B)square plane.
C)pyramid.
D)trigonal plane.
E)bent structure.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
41
What is the H-C-C bond angle in ethylene,H2CCH2?
A)slightly less than 120°
B)90°
C)109°
D)120°
E)180°
A)slightly less than 120°
B)90°
C)109°
D)120°
E)180°

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following molecules is nonpolar?
A)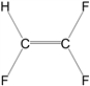
B)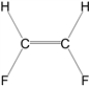
C)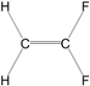
D)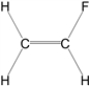
E)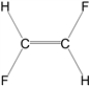
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
43
What is the H-O-H bond angle in water?
A)90°
B)slightly less than 109°
C)180°
D)120°
E)109°
A)90°
B)slightly less than 109°
C)180°
D)120°
E)109°

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following statements correctly describes the reaction of BF3 with NH3 to form F3B-NH3?
A)Both nitrogen and boron change from trigonal planar to tetrahedral geometry during the reaction.
B)Boron changes from trigonal planar to tetrahedral geometry during the reaction.
C)There are no changes in the formal charge on any atom during the reaction.
D)Nitrogen changes from trigonal planar to tetrahedral geometry during the reaction.
E)There is no change in geometry around the nitrogen or boron atoms.
A)Both nitrogen and boron change from trigonal planar to tetrahedral geometry during the reaction.
B)Boron changes from trigonal planar to tetrahedral geometry during the reaction.
C)There are no changes in the formal charge on any atom during the reaction.
D)Nitrogen changes from trigonal planar to tetrahedral geometry during the reaction.
E)There is no change in geometry around the nitrogen or boron atoms.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following molecules has a permanent dipole moment?
A)SF6
B)CCl4
C)NF3
D)SiCl4
E)BF3
A)SF6
B)CCl4
C)NF3
D)SiCl4
E)BF3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
46
What is the molecular geometry around oxygen atom O1?
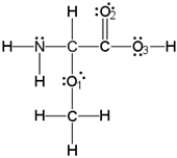
A)bent
B)tetrahedral
C)see-saw or distorted tetrahedral
D)trigonal planar
E)T-shaped

A)bent
B)tetrahedral
C)see-saw or distorted tetrahedral
D)trigonal planar
E)T-shaped

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
47
Which molecule or ion does not have a tetrahedral molecular geometry?
A)BF4-
B)NF4+
C)GeF4
D)XeF4
E)BeF42-
A)BF4-
B)NF4+
C)GeF4
D)XeF4
E)BeF42-

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
48
In the ICI4- ion,how many electron groups surround the central iodine atom?
A)2
B)4
C)5
D)6
E)3
A)2
B)4
C)5
D)6
E)3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
49
Which molecule or ion does not have a tetrahedral molecular geometry?
A)ICl4-
B)CCl4
C)GeCl4
D)BrO4-
E)SiCl4
A)ICl4-
B)CCl4
C)GeCl4
D)BrO4-
E)SiCl4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following molecules does not have a permanent dipole moment?
A)iodine trichloride,ICl3
B)nitrogen trifluoride,NF3
C)sulfur dioxide,SO2
D)carbon tetrachloride,CCl4
E)hydrogen fluoride,HF
A)iodine trichloride,ICl3
B)nitrogen trifluoride,NF3
C)sulfur dioxide,SO2
D)carbon tetrachloride,CCl4
E)hydrogen fluoride,HF

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
51
In the I3- ion,how many electron groups surround the central atom?
A)5
B)3
C)6
D)4
E)2
A)5
B)3
C)6
D)4
E)2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
52
What is the molecular geometry of the bromate ion,BrO3-?
A)square planar
B)trigonal planar
C)square pyramidal
D)tetrahedral
E)trigonal pyramidal
A)square planar
B)trigonal planar
C)square pyramidal
D)tetrahedral
E)trigonal pyramidal

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
53
Which molecule or ion does not have a tetrahedral molecular geometry?
A)ClF4-
B)SiF4
C)NF4+
D)CF4
E)BF4-
A)ClF4-
B)SiF4
C)NF4+
D)CF4
E)BF4-

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following molecules has a dipole moment?
A)PF5
B)FOOF
C)HCCH
D)F2CCF2
E)SF6
A)PF5
B)FOOF
C)HCCH
D)F2CCF2
E)SF6

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
55
Which molecule is polar?
A)C2H4
B)CS2
C)C6H6
D)SO2
E)CF4
A)C2H4
B)CS2
C)C6H6
D)SO2
E)CF4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
56
What is the molecular geometry of the ICl4- ion?
A)octahedral
B)pentagonal
C)tetrahedral
D)rectangular
E)square planar
A)octahedral
B)pentagonal
C)tetrahedral
D)rectangular
E)square planar

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
57
In ClF3,the electron pairs are arranged about the chlorine atom in
A)a square plane.
B)a tetrahedron.
C)an octahedron.
D)a trigonal pyramid.
E)a trigonal bipyramid.
A)a square plane.
B)a tetrahedron.
C)an octahedron.
D)a trigonal pyramid.
E)a trigonal bipyramid.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
58
In the ICl4- ion,the electron pairs are arranged around the central iodine atom in the shape of
A)a tetrahedron.
B)an octahedron.
C)a square plane.
D)a trigonal bipyramid.
E)a trigonal pyramid.
A)a tetrahedron.
B)an octahedron.
C)a square plane.
D)a trigonal bipyramid.
E)a trigonal pyramid.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following characteristics does not apply to PF3?
A)has three σ bonds
B)contains polar bonds
C)polar molecule
D)one lone pair of electrons on phosphorus
E)trigonal planar
A)has three σ bonds
B)contains polar bonds
C)polar molecule
D)one lone pair of electrons on phosphorus
E)trigonal planar

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
60
For which molecule or ion does the nitrogen atom have the positive end of the dipole moment?
A)NH4+
B)CN−
C)NO
D)HCN
E)N2
A)NH4+
B)CN−
C)NO
D)HCN
E)N2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
61
What is the hybridization of Se in SeF6?
A)sp3d
B)sp3d2
C)sp2
D)sp
E)sp3
A)sp3d
B)sp3d2
C)sp2
D)sp
E)sp3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
62
What is the hybridization of Br in BrF3?
A)sp3
B)sp
C)sp3d2
D)sp2
E)sp3d
A)sp3
B)sp
C)sp3d2
D)sp2
E)sp3d

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
63
Which one of the following statements provides the best evidence that all the carbon compounds listed below have tetrahedral molecular geometries instead of square planar molecular geometries?
A)Only one CH4 compound is known and it is nonpolar.
B)Only one CH3F compound is known and it is polar.
C)Only one CH2F2 compound is known and it is polar.
D)Only one CF4 compound is known and it is nonpolar.
E)Only one CHF3 compound is known and it is polar.
A)Only one CH4 compound is known and it is nonpolar.
B)Only one CH3F compound is known and it is polar.
C)Only one CH2F2 compound is known and it is polar.
D)Only one CF4 compound is known and it is nonpolar.
E)Only one CHF3 compound is known and it is polar.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
64
According to valence-bond theory,what is the hybridization scheme of the sulfur atom in SF4?
A)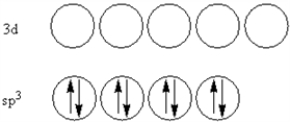
B)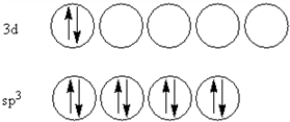
C)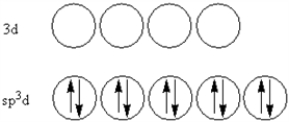
D)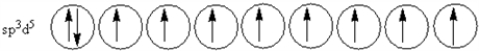
E)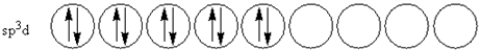
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
65
What is the hybridization of the nitrogen atom in the nitrite ion?
A)sp3d
B)sp3
C)s
D)sp
E)sp2
A)sp3d
B)sp3
C)s
D)sp
E)sp2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
66
What hybrid orbitals of sulfur are involved in the bonding in sulfur trioxide?
A)sp2
B)sp2d
C)sp3
D)sp3d2
E)sp
A)sp2
B)sp2d
C)sp3
D)sp3d2
E)sp

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following compounds is nonpolar?
A)H2S
B)XeF2
C)SO2
D)N2O
E)HCl
A)H2S
B)XeF2
C)SO2
D)N2O
E)HCl

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following molecules is polar?
A)SF6
B)CCl4
C)BF3
D)NO2
E)CO2
A)SF6
B)CCl4
C)BF3
D)NO2
E)CO2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following statements is true?
A)A π bond is twice as strong as a single bond.
B)A π bond results from the sideways overlap of hybridized orbitals.
C)A double bond consists of a π bond and a σ bond.
D)A π bond has cylindrical symmetry about the bonding axis.
E)A π bond is twice as strong as a σ bond.
A)A π bond is twice as strong as a single bond.
B)A π bond results from the sideways overlap of hybridized orbitals.
C)A double bond consists of a π bond and a σ bond.
D)A π bond has cylindrical symmetry about the bonding axis.
E)A π bond is twice as strong as a σ bond.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
70
A π bond is the result of the
A)overlap of two s orbitals.
B)overlap of two p orbitals along their axes.
C)sideways overlap of two parallel p orbitals.
D)overlap of an s orbital and a p orbital.
E)sideways overlap of two s orbitals.
A)overlap of two s orbitals.
B)overlap of two p orbitals along their axes.
C)sideways overlap of two parallel p orbitals.
D)overlap of an s orbital and a p orbital.
E)sideways overlap of two s orbitals.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
71
What is the hybridization of I in IF4-?
A)sp2
B)sp
C)sp3d
D)sp3d2
E)sp3
A)sp2
B)sp
C)sp3d
D)sp3d2
E)sp3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following molecules has a dipole moment?
A)SO2
B)CS2
C)ClCCCCCl
D)CCl4
E)HCCH
A)SO2
B)CS2
C)ClCCCCCl
D)CCl4
E)HCCH

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
73
When an atom in a molecule or ion is described as sp3d1 hybridized,its molecular geometry is
A)trigonal bipyramidal.
B)trigonal planar.
C)linear.
D)tetrahedral.
E)octahedral.
A)trigonal bipyramidal.
B)trigonal planar.
C)linear.
D)tetrahedral.
E)octahedral.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
74
Which molecule or ion does not contain two π bonds?
A)HCCH
B)H2CCCH2
C)NO-
D)CS2
E)SCN-
A)HCCH
B)H2CCCH2
C)NO-
D)CS2
E)SCN-

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
75
How many sigma and pi bonds are in the molecule pictured below? 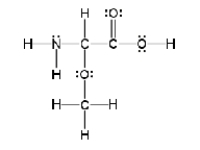
A)thirteen sigma bonds and one pi bond
B)eleven sigma bonds and two pi bonds
C)thirteen sigma bonds and two pi bonds
D)eleven sigma bonds and five pi bonds
E)five sigma bonds and eleven pi bonds

A)thirteen sigma bonds and one pi bond
B)eleven sigma bonds and two pi bonds
C)thirteen sigma bonds and two pi bonds
D)eleven sigma bonds and five pi bonds
E)five sigma bonds and eleven pi bonds

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following molecules is nonpolar?
A)SF4
B)PF5
C)ClF3
D)PF3
E)CH2F2
A)SF4
B)PF5
C)ClF3
D)PF3
E)CH2F2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following statements is incorrect regarding the water molecule?
A)There are two lone pairs and two bonding pairs on the central atom.
B)The molecule is polar.
C)The hybridization of oxygen is sp3.
D)The hybridization of hydrogen is sp.
E)The molecular geometry is bent.
A)There are two lone pairs and two bonding pairs on the central atom.
B)The molecule is polar.
C)The hybridization of oxygen is sp3.
D)The hybridization of hydrogen is sp.
E)The molecular geometry is bent.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following statements best describes N2O?
A)The molecular geometry is bent and the molecule is nonpolar.
B)The molecular geometry is linear and the molecule is nonpolar.
C)The molecular geometry is linear and the molecule is polar.
D)The molecular geometry is trigonal planar and the molecule is nonpolar.
E)The molecular geometry is bent and the molecule is polar.
A)The molecular geometry is bent and the molecule is nonpolar.
B)The molecular geometry is linear and the molecule is nonpolar.
C)The molecular geometry is linear and the molecule is polar.
D)The molecular geometry is trigonal planar and the molecule is nonpolar.
E)The molecular geometry is bent and the molecule is polar.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the labeled carbons (C1-C4)is/are sp-hybridized?
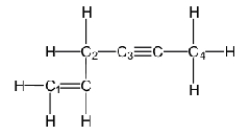
A)carbon three
B)carbon one
C)carbon two and four
D)carbon two
E)carbon one and three

A)carbon three
B)carbon one
C)carbon two and four
D)carbon two
E)carbon one and three

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
80
According to valence-bond theory,the bonding in ketene,H2CCO,is best described as
A)five π bonds.
B)three σ bonds and two π bonds.
C)four σ bonds and two π bonds.
D)four σ bonds and one π bond.
E)five σ bonds.
A)five π bonds.
B)three σ bonds and two π bonds.
C)four σ bonds and two π bonds.
D)four σ bonds and one π bond.
E)five σ bonds.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck


