Exam 10: Molecular Geometry and Chemical Bonding Theory
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
What is the molecular geometry of the bromate ion,BrO3-?
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
E
What is the electron geometry (or electron arrangement)around an atom in a molecule or ion which is surrounded by zero lone pairs of electrons and six single bonds.
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
A
Which molecule or ion does not have a planar molecular geometry?
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
E
Which of the following species has(have)a bond order of 1?
1)HF
2)O22−
3)O22+
(Multiple Choice)
4.9/5  (40)
(40)
Which molecule or ion has the same molecular geometry for its central atom as the carbonate ion?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following electron distributions among the molecular orbitals best describes the NO molecule?
Σ2s
Σ2s*
Π2py=π2px
Σ2pz
Π2py*=π2px*
Σ2pz*
(Multiple Choice)
4.7/5  (34)
(34)
Which one of the following statements provides the best evidence that all the carbon compounds listed below have tetrahedral molecular geometries instead of square planar molecular geometries?
(Multiple Choice)
4.9/5  (36)
(36)
What is the molecular geometry around an atom in a molecule or ion which is surrounded by two lone pairs of electrons and four single bonds.
(Multiple Choice)
4.9/5  (45)
(45)
Consider the following series of molecular ions and molecules: F2+,F22+,F2,and F2-.Which will have the shortest bond length between the fluorine atoms? Assume the homonuclear molecular orbital diagram provided below for nitrogen (excluding the K shells)still applies to these species. 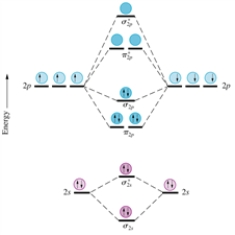
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following molecules has a permanent dipole moment?
(Multiple Choice)
4.7/5  (27)
(27)
In phosgene,COCl2,the electron groups are located about the central carbon atom in a ______________ arrangement.
(Multiple Choice)
4.7/5  (39)
(39)
What is the molecular geometry around an atom in a molecule or ion which is surrounded by five single bonds and no lone pairs of electrons.
(Multiple Choice)
4.9/5  (41)
(41)
In the ICI4- ion,how many electron groups surround the central iodine atom?
(Multiple Choice)
4.8/5  (41)
(41)
According to valence-bond theory,what is the hybridization scheme of the sulfur atom in SF4?
(Multiple Choice)
4.8/5  (40)
(40)
Showing 1 - 20 of 101
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)