Deck 11: States of Matter; Liquids and Solids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/123
Play
Full screen (f)
Deck 11: States of Matter; Liquids and Solids
1
The boiling point of a liquid is
A)always the temperature at which the vapor pressure equals 760 mmHg (1 atm).
B)always the temperature at which the liquid phase of a substance is in equilibrium with the vapor phase.
C)always the temperature at which the vapor pressure equals the pressure exerted on the liquid.
D)always equal to the vapor pressure of the liquid at a given temperature.
E)independent of the pressure exerted on the liquid.
A)always the temperature at which the vapor pressure equals 760 mmHg (1 atm).
B)always the temperature at which the liquid phase of a substance is in equilibrium with the vapor phase.
C)always the temperature at which the vapor pressure equals the pressure exerted on the liquid.
D)always equal to the vapor pressure of the liquid at a given temperature.
E)independent of the pressure exerted on the liquid.
always the temperature at which the vapor pressure equals the pressure exerted on the liquid.
2
Which of the following involves a change in temperature during the phase transition?
A)condensation of water
B)liquifaction of ammonia
C)fusion of ethanol
D)all of the above
E)none of the above
A)condensation of water
B)liquifaction of ammonia
C)fusion of ethanol
D)all of the above
E)none of the above
none of the above
3
Assume 12,500 J of energy is added to 2.0 moles (36 grams)of H2O as an ice sample at 0°C.The molar heat of fusion is 6.02 kJ/mol.The specific heat of liquid water is 4.18 J/g °C.The molar heat of vaporization is 40.6 kJ/mol.The resulting sample contains which of the following?
A)water and water vapor
B)ice and water
C)only water
D)only water vapor
E)only ice
A)water and water vapor
B)ice and water
C)only water
D)only water vapor
E)only ice
only water
4
The process represented by the equation C10H8(s)→ C10H8(g)is
A)melting.
B)liquefaction.
C)sublimation.
D)condensation.
E)fusion.
A)melting.
B)liquefaction.
C)sublimation.
D)condensation.
E)fusion.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
5
The vapor pressure of a given liquid will increase if
A)the liquid is moved to a container in which its surface is very much larger.
B)the volume of the liquid is increased.
C)the temperature is increased.
D)the volume of the vapor phase is increased.
E)a more volatile liquid is added to the given liquid.
A)the liquid is moved to a container in which its surface is very much larger.
B)the volume of the liquid is increased.
C)the temperature is increased.
D)the volume of the vapor phase is increased.
E)a more volatile liquid is added to the given liquid.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
6
A particular compound has an enthalpy of vaporization of 28400 J/mol.At 274 K it has a vapor pressure of 122 mmHg.What is its vapor pressure at 311 K? (R = 8.31 J/(K· mol))
A)117 mmHg
B)262 mmHg
C)27.6 mmHg
D)538 mmHg
E)126 mmHg
A)117 mmHg
B)262 mmHg
C)27.6 mmHg
D)538 mmHg
E)126 mmHg

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
7
A liquid has an enthalpy of vaporization of 29.1 kJ/mol.At 274 K it has a vapor pressure of 103 mmHg.What is the normal boiling point of this liquid? (R = 8.31 J/(K· mol))
A)294 K
B)325 K
C)274 K
D)257 K
E)237 K
A)294 K
B)325 K
C)274 K
D)257 K
E)237 K

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
8
In a certain mountain range,water boils at 94°C.What is the atmospheric pressure under these conditions? The enthalpy of vaporization of water at 100°C is 40.7 kJ/mol.(R = 8.31 J/(K · mol))
A)1750 mmHg
B)324 mmHg
C)613 mmHg
D)941 mmHg
E)329 mmHg
A)1750 mmHg
B)324 mmHg
C)613 mmHg
D)941 mmHg
E)329 mmHg

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
9
When a solid undergoes a phase change to a gas,the process is called
A)fusion.
B)condensation.
C)melting.
D)vaporization.
E)sublimation.
A)fusion.
B)condensation.
C)melting.
D)vaporization.
E)sublimation.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
10
In which of the following processes will energy be evolved as heat?
A)crystallization
B)melting
C)sublimation
D)vaporization
E)none of these
A)crystallization
B)melting
C)sublimation
D)vaporization
E)none of these

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following processes is exothermic?
A)condensation of water
B)fusion of ethanol
C)vaporization of water
D)evaporation of ammonia
E)sublimation of carbon dioxide
A)condensation of water
B)fusion of ethanol
C)vaporization of water
D)evaporation of ammonia
E)sublimation of carbon dioxide

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
12
What is the name for the following phase change?
I2(l)→ I2(g)
A)vaporization
B)freezing
C)condensation
D)sublimation
E)melting
I2(l)→ I2(g)
A)vaporization
B)freezing
C)condensation
D)sublimation
E)melting

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
13
A bottle is filled with a small amount of a volatile liquid and sealed.Sometime later it is observed that no liquid is evident in the sealed bottle.Which of the following statements would explain this observation?
A)More time is needed to establish equilibrium.
B)Liquid and vapor are at equilibrium in the bottle.
C)Too little liquid was added to achieve a liquid vapor equilibrium in the closed system.
D)The vapor state is favored when equilibrium is established.
E)The liquid has undergone sublimation.
A)More time is needed to establish equilibrium.
B)Liquid and vapor are at equilibrium in the bottle.
C)Too little liquid was added to achieve a liquid vapor equilibrium in the closed system.
D)The vapor state is favored when equilibrium is established.
E)The liquid has undergone sublimation.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
14
If more ice is added to an ice-water mixture at equilibrium,
A)the temperature will increase somewhat.
B)the vapor pressure of the water will decrease.
C)the temperature will decrease somewhat.
D)the vapor pressure of the water will rise.
E)the vapor pressure of the water will remain constant.
A)the temperature will increase somewhat.
B)the vapor pressure of the water will decrease.
C)the temperature will decrease somewhat.
D)the vapor pressure of the water will rise.
E)the vapor pressure of the water will remain constant.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
15
The vapor pressure of a liquid increases with increasing temperature.Which of the following statements best explains this relationship?
A)All the molecules have greater kinetic energies.
B)The number of gaseous molecules above the liquid remains constant,but these molecules have greater average kinetic energy.
C)The faster-moving molecules in the liquid exert a greater pressure.
D)The intermolecular forces between the molecules decrease at higher temperatures.
E)The average kinetic energy of molecules is greater; thus more molecules can enter the gaseous state.
A)All the molecules have greater kinetic energies.
B)The number of gaseous molecules above the liquid remains constant,but these molecules have greater average kinetic energy.
C)The faster-moving molecules in the liquid exert a greater pressure.
D)The intermolecular forces between the molecules decrease at higher temperatures.
E)The average kinetic energy of molecules is greater; thus more molecules can enter the gaseous state.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
16
What is the value of q when 8.29 g of water vaporizes at 373 K? The enthalpy of condensation of water at 373 K is -40.7 kJ/mol.
A)-337 kJ
B)-18.7 kJ
C)18.7 kJ
D)337 kJ
E)0.203 kJ
A)-337 kJ
B)-18.7 kJ
C)18.7 kJ
D)337 kJ
E)0.203 kJ

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following phase changes are endothermic?
A)sublimation
B)condensation
C)deposition
D)liquifaction
E)crystallization
A)sublimation
B)condensation
C)deposition
D)liquifaction
E)crystallization

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
18
The enthalpy of fusion of aluminum is 10.7 kJ/mol.How many grams of aluminum can be melted by adding 81.4 kJ of energy to the metal at its melting point?
A)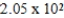 g
g
B)32.2g
C)3.01 g
D)7.6 g
E)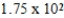 g
g
A)
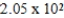 g
gB)32.2g
C)3.01 g
D)7.6 g
E)
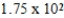 g
g
Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following statements concerning liquids is incorrect?
A)The volume of a liquid changes very little with pressure.
B)Liquids are relatively incompressible.
C)Liquid molecules move slowly compared to solids.
D)Non-volatile liquids have low vapor pressures at room temperature.
E)The molecules of a liquid are in constant random motion.
A)The volume of a liquid changes very little with pressure.
B)Liquids are relatively incompressible.
C)Liquid molecules move slowly compared to solids.
D)Non-volatile liquids have low vapor pressures at room temperature.
E)The molecules of a liquid are in constant random motion.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
20
How much heat is released at constant pressure if a 15.0-L tank containing 47.0 atm of hydrogen sulfide gas condenses at its boiling point of -60.0oC? The enthalpy of vaporization of hydrogen sulfide is 18.7 kJ/mol at -60.0oC.(R = 0.0821 L • atm/(K • mol))
A)1.31 × 102 J
B)2.68 × 106 J
C)4.64× 102 J
D)1.87× 104 J
E)7.54 × 105 J
A)1.31 × 102 J
B)2.68 × 106 J
C)4.64× 102 J
D)1.87× 104 J
E)7.54 × 105 J

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
21
Which explanation best describes surface tension?
A)Molecules at the surface of a liquid experience a net force towards the liquid's interior.
B)Molecules at the edges of a liquid adhere to the surface of the liquid's container.
C)Molecules of a liquid tend to form a concave meniscus.
D)Molecules of a liquid tend to resist flow.
E)Molecules of a liquid have a very low vapor pressure.
A)Molecules at the surface of a liquid experience a net force towards the liquid's interior.
B)Molecules at the edges of a liquid adhere to the surface of the liquid's container.
C)Molecules of a liquid tend to form a concave meniscus.
D)Molecules of a liquid tend to resist flow.
E)Molecules of a liquid have a very low vapor pressure.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
22
From a consideration of the phase diagram below,a change from point M to point N corresponds to 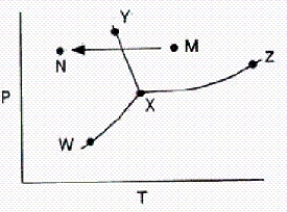
A)sublimation.
B)liquefaction.
C)evaporation.
D)condensation.
E)freezing.

A)sublimation.
B)liquefaction.
C)evaporation.
D)condensation.
E)freezing.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
23
The critical point of CCl4 is 283°C and 45 atm pressure.Liquid CCl4 has a vapor pressure of 10.0 atm at 178°C.Which of the following statements must be true?
A)Vapor and liquid can only be in equilibrium at one temperature-the normal boiling point.
B)Liquid CCl4 can exist at temperatures greater than 283°C if the pressure is greater than 45 atm.
C)Liquid and solid can only be in equilibrium at one temperature-the freezing point.
D)The triple point of CCl4 must be less than 178°C.
E)The normal boiling point of CCl4 must be greater than 178°C.
A)Vapor and liquid can only be in equilibrium at one temperature-the normal boiling point.
B)Liquid CCl4 can exist at temperatures greater than 283°C if the pressure is greater than 45 atm.
C)Liquid and solid can only be in equilibrium at one temperature-the freezing point.
D)The triple point of CCl4 must be less than 178°C.
E)The normal boiling point of CCl4 must be greater than 178°C.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
24
The triple point of iodine is at 90 torr and 115°C.This means that liquid I2
A)cannot have a vapor pressure less than 90 torr.
B)is more dense than I2(s).
C)cannot exist at 1 atmosphere pressure.
D)cannot exist above 115°C.
E)can exist at pressure of 10 torr.
A)cannot have a vapor pressure less than 90 torr.
B)is more dense than I2(s).
C)cannot exist at 1 atmosphere pressure.
D)cannot exist above 115°C.
E)can exist at pressure of 10 torr.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
25
Choose the correct statement about the diagram below. 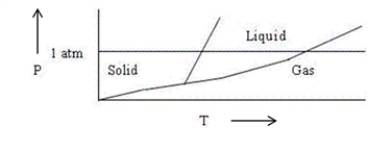
A)The diagram shows the triple point above 1 atm pressure.
B)The diagram is qualitatively correct for water.
C)The diagram shows that the melting point of the solid increases with increasing pressure.
D)The diagram could represent the phase diagram of CO2.
E)None of the above statements is correct.

A)The diagram shows the triple point above 1 atm pressure.
B)The diagram is qualitatively correct for water.
C)The diagram shows that the melting point of the solid increases with increasing pressure.
D)The diagram could represent the phase diagram of CO2.
E)None of the above statements is correct.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
26
In which of the following substances are intermolecular forces of attraction absent?
A)HF(l)
B)CCl4(l)
C)NaCl(l)
D)H2O(l)
E)N2(l)
A)HF(l)
B)CCl4(l)
C)NaCl(l)
D)H2O(l)
E)N2(l)

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
27
If the liquid of a pure substance has a lower density than the solid,what is the effect on the pressure-temperature phase diagram?
A)The vapor-pressure curve arches upward.
B)The normal melting point is above room temperature.
C)The melting-point curve has a negative slope.
D)The vapor-pressure curve arches downward.
E)The melting-point curve has a positive slope.
A)The vapor-pressure curve arches upward.
B)The normal melting point is above room temperature.
C)The melting-point curve has a negative slope.
D)The vapor-pressure curve arches downward.
E)The melting-point curve has a positive slope.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
28
If the diameter of a spherical water droplet is 100.0 μm,how much energy is required to increase the diameter of the water droplet by 5.0 μm? The surface tension of water is 1.0 × 10-3 J/m2.
A)3.1 × 10-11 J
B)3 × 10-12 J
C)7.9 × 10-14 J
D)1 × 10-11 J
E)4 × 10-11 J
A)3.1 × 10-11 J
B)3 × 10-12 J
C)7.9 × 10-14 J
D)1 × 10-11 J
E)4 × 10-11 J

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
29
What is the enthalpy of vaporization of a compound that has a vapor pressure of 151 mmHg at 247 K and 1.52 mmHg at 187 K? (R = 8.31 J/(K· mol))
A)636 kJ/mol
B)2 kJ/mol
C)4 kJ/mol
D)29 kJ/mol
E)290 kJ/mol
A)636 kJ/mol
B)2 kJ/mol
C)4 kJ/mol
D)29 kJ/mol
E)290 kJ/mol

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following statements concerning the accompanying phase diagram is false? 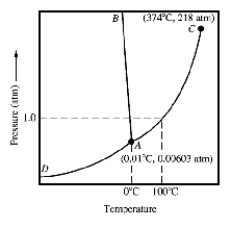
A)The solid is more dense than the liquid.
B)Point C is the critical point of the substance.
C)Point A is the triple point of the substance.
D)The normal boiling point is above the triple point.
E)The curve AD divides the solid region from the gas region.

A)The solid is more dense than the liquid.
B)Point C is the critical point of the substance.
C)Point A is the triple point of the substance.
D)The normal boiling point is above the triple point.
E)The curve AD divides the solid region from the gas region.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
31
Below is a phase diagram for a substance. 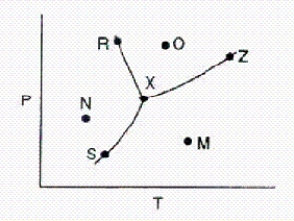 Which line represents the melting-point curve of the substance?
Which line represents the melting-point curve of the substance?
A)R-X
B)S-X
C)X-Z
D)S-Z
E)M-N
 Which line represents the melting-point curve of the substance?
Which line represents the melting-point curve of the substance?A)R-X
B)S-X
C)X-Z
D)S-Z
E)M-N

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
32
Below is a phase diagram for a substance. 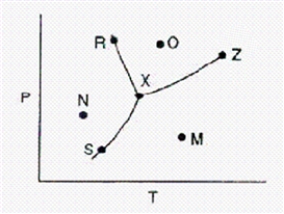 What is the name for point Z on the diagram?
What is the name for point Z on the diagram?
A)normal boiling point.
B)critical point.
C)melting point.
D)boiling point.
E)triple point.
 What is the name for point Z on the diagram?
What is the name for point Z on the diagram?A)normal boiling point.
B)critical point.
C)melting point.
D)boiling point.
E)triple point.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
33
Which is the best reason for why water in a glass capillary has a concave meniscus,while mercury in a glass capillary has a convex meniscus?
A)Mercury has a greater dispersion force than water.
B)The water is attracted more strongly to the glass than the mercury is attracted to the glass.
C)The mercury is attracted more strongly to the glass than the water is attracted to the glass.
D)Water is a molecular compound while mercury is a metallic element.
E)Water has a greater dispersion force than mercury.
A)Mercury has a greater dispersion force than water.
B)The water is attracted more strongly to the glass than the mercury is attracted to the glass.
C)The mercury is attracted more strongly to the glass than the water is attracted to the glass.
D)Water is a molecular compound while mercury is a metallic element.
E)Water has a greater dispersion force than mercury.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
34
The measure of the resistance to flow of a liquid is
A)London forces.
B)van der Waals forces.
C)viscosity.
D)vapor pressure.
E)surface tension.
A)London forces.
B)van der Waals forces.
C)viscosity.
D)vapor pressure.
E)surface tension.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
35
Knowing that ΔHvap for water is 40.7 kJ/mol,calculate Pvap of water at 37°C.
A)52.7 torr
B)25.4 torr
C)18.7 torr
D)12.4 torr
E)6.90 torr
A)52.7 torr
B)25.4 torr
C)18.7 torr
D)12.4 torr
E)6.90 torr

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
36
For a particular liquid,raising its temperature from 339 K to 365 K causes its vapor pressure to double.What is the enthalpy of vaporization of this liquid? (R = 8.31 J/(K · mol))
A)27 kJ/mol
B)221 kJ/mol
C)3 kJ/mol
D)307 kJ/mol
E)149 kJ/mol
A)27 kJ/mol
B)221 kJ/mol
C)3 kJ/mol
D)307 kJ/mol
E)149 kJ/mol

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
37
Below is a phase diagram for a substance. 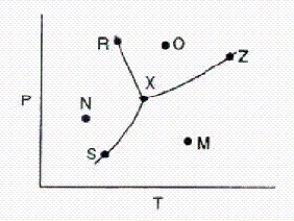 Which line represents the vapor-pressure curve of the substance?
Which line represents the vapor-pressure curve of the substance?
A)S-Z
B)X-Z
C)S-X
D)M-N
E)R-X
 Which line represents the vapor-pressure curve of the substance?
Which line represents the vapor-pressure curve of the substance?A)S-Z
B)X-Z
C)S-X
D)M-N
E)R-X

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
38
Below is a phase diagram for a substance. 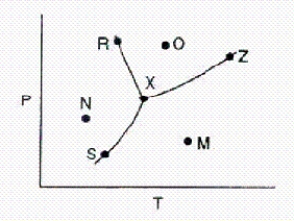 What is the name for point X on the diagram?
What is the name for point X on the diagram?
A)boiling point
B)normal boiling point
C)triple point
D)melting point
E)critical point
 What is the name for point X on the diagram?
What is the name for point X on the diagram?A)boiling point
B)normal boiling point
C)triple point
D)melting point
E)critical point

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
39
Given the accompanying phase diagram,under what conditions will solid be found in equilibrium with either liquid or gas? 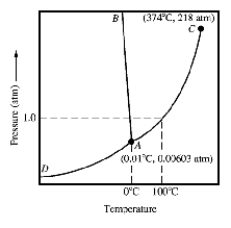
A)Anywhere along curve AB.
B)Anywhere along curve AC.
C)Anywhere along curve AD.
D)Anywhere along curve AB and AD.
E)Anywhere along curve AC and AD.

A)Anywhere along curve AB.
B)Anywhere along curve AC.
C)Anywhere along curve AD.
D)Anywhere along curve AB and AD.
E)Anywhere along curve AC and AD.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
40
In the accompanying phase diagram,a liquid can be present at combinations of temperature and pressure corresponding to points 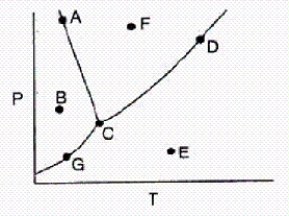

A)A,C,G,and D.
B)A,C,D,and F.
C)A,B,C,and G.
D)A and C only.
E)G,C,D,and E.


A)A,C,G,and D.
B)A,C,D,and F.
C)A,B,C,and G.
D)A and C only.
E)G,C,D,and E.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following indicates the existence of strong intermolecular forces of attraction in a liquid?
A)a very low critical temperature
B)a very low boiling point
C)a very low vapor pressure
D)a very low viscosity
E)a very low enthalpy of vaporization
A)a very low critical temperature
B)a very low boiling point
C)a very low vapor pressure
D)a very low viscosity
E)a very low enthalpy of vaporization

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following pure substances has the highest normal boiling point?
A)HI
B)HCl
C)HF
D)H2S
E)HBr
A)HI
B)HCl
C)HF
D)H2S
E)HBr

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
43
Which compound has the lowest standard enthalpy of vaporization at 25°C?
A)C6H14
B)C8H16
C)C5H12
D)C8H18
E)C7H16
A)C6H14
B)C8H16
C)C5H12
D)C8H18
E)C7H16

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following pure substances has an unusually high normal boiling point?
A)CH3OCH3
B)CH3SH
C)HCl
D)CH3NH2
E)CH3Cl
A)CH3OCH3
B)CH3SH
C)HCl
D)CH3NH2
E)CH3Cl

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
45
In an experiment,40.0 mmol of helium gas is collected over water.The total volume of gas collected is 0.224 L.Under similar conditions,the gas is collected over two other liquids,A and B.The total volume of gas collected over A and B are 0.222 L and 0.227 L,respectively.Which of the following statements is false?
A)Liquid B boils at a higher temperature than water
B)Liquid A boils at a higher temperature than water
C)The vapor pressure of B is higher than that of A
D)The vapor pressure of B is higher than that of water
E)Liquid A boils at a temperature higher than B
A)Liquid B boils at a higher temperature than water
B)Liquid A boils at a higher temperature than water
C)The vapor pressure of B is higher than that of A
D)The vapor pressure of B is higher than that of water
E)Liquid A boils at a temperature higher than B

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following pure substances has the highest standard enthalpy of vaporization at 25°C?
A)H2O
B)NH3
C)PH3
D)AsH3
E)SbH3
A)H2O
B)NH3
C)PH3
D)AsH3
E)SbH3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following compounds has the highest normal boiling point?
A)CH3CH2CH2CH3
B)CH3Cl
C)CH3CH2OH
D)CH3OCH3
E)CH3CH2CH3
A)CH3CH2CH2CH3
B)CH3Cl
C)CH3CH2OH
D)CH3OCH3
E)CH3CH2CH3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following pure substances has the lowest normal boiling point?
A)H2S
B)NH3
C)H2O
D)H2Te
E)H2Se
A)H2S
B)NH3
C)H2O
D)H2Te
E)H2Se

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
49
Rank the following molecules in order of increasing normal boiling point: CH3CH2OH,CH3CH2CH2OH,CH3CH2OCH3.
A)lowest CH3CH2CH2OH,CH3CH2OCH3,CH3CH2OH highest.
B)lowest CH3CH2OH,CH3CH2OCH3,CH3CH2CH2OH highest.
C)lowest CH3CH2CH2OH,CH3CH2OH,CH3CH2OCH3 highest.
D)lowest CH3CH2OCH3,CH3CH2OH,CH3CH2CH2OH highest.
E)lowest CH3CH2OCH3,CH3CH2CH2OH,CH3CH2OH highest.
A)lowest CH3CH2CH2OH,CH3CH2OCH3,CH3CH2OH highest.
B)lowest CH3CH2OH,CH3CH2OCH3,CH3CH2CH2OH highest.
C)lowest CH3CH2CH2OH,CH3CH2OH,CH3CH2OCH3 highest.
D)lowest CH3CH2OCH3,CH3CH2OH,CH3CH2CH2OH highest.
E)lowest CH3CH2OCH3,CH3CH2CH2OH,CH3CH2OH highest.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
50
Why does hydrogen fluoride have an unusually high normal boiling point compared to the other hydrogen halides?
A)The H-F bond in hydrogen fluoride is very strong.
B)Hydrogen fluoride has very strong London dispersion forces.
C)Hydrogen fluoride is capable of forming hydrogen bonds.
D)Hydrogen fluoride is ionic.
E)Hydrogen fluoride is covalent.
A)The H-F bond in hydrogen fluoride is very strong.
B)Hydrogen fluoride has very strong London dispersion forces.
C)Hydrogen fluoride is capable of forming hydrogen bonds.
D)Hydrogen fluoride is ionic.
E)Hydrogen fluoride is covalent.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
51
At 25°C,the vapor pressure of diethyl ether,(CH3CH2)2O,is higher than the vapor pressure of its isomer n-butanol,CH3CH2CH2CH2OH,because
A)diethyl ether has a higher density than n-butanol.
B)diethyl ether has weaker intermolecular forces than n-butanol.
C)diethyl ether has a lower critical temperature than n-butanol.
D)diethyl ether has a higher surface tension than n-butanol.
E)diethyl ether has weaker intramolecular forces than n-butanol.
A)diethyl ether has a higher density than n-butanol.
B)diethyl ether has weaker intermolecular forces than n-butanol.
C)diethyl ether has a lower critical temperature than n-butanol.
D)diethyl ether has a higher surface tension than n-butanol.
E)diethyl ether has weaker intramolecular forces than n-butanol.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
52
When two water molecule form a hydrogen bond,which atoms are involved in the interaction?
A)Two hydrogens from one molecule and one hydrogen from the other molecule
B)An oxygen from one molecule and an oxygen from the other molecule
C)Two hydrogens from one molecule and one oxygen from the other molecule
D)A hydrogen from one molecule and a hydrogen from the other molecule
E)A hydrogen from one molecule and an oxygen from the other molecule
A)Two hydrogens from one molecule and one hydrogen from the other molecule
B)An oxygen from one molecule and an oxygen from the other molecule
C)Two hydrogens from one molecule and one oxygen from the other molecule
D)A hydrogen from one molecule and a hydrogen from the other molecule
E)A hydrogen from one molecule and an oxygen from the other molecule

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following pure substances has the highest vapor pressure at room temperature?
A)Si3H8
B)Si2H6
C)Si2Cl6
D)Si4H10
E)SiH4
A)Si3H8
B)Si2H6
C)Si2Cl6
D)Si4H10
E)SiH4

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
54
Sulfur trioxide (SO3)is able to be liquefied at low temperatures due to which intermolecular force?
A)ionic bonding
B)covalent bonding
C)hydrogen bonding
D)dipole-dipole
E)London dispersion
A)ionic bonding
B)covalent bonding
C)hydrogen bonding
D)dipole-dipole
E)London dispersion

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following compounds has the highest vapor pressure at 25°C?
A)CH3CH2OH
B)CH3CH2CH2CH3
C)CH3OCH3
D)CH3CH2CH3
E)CH3CH2CH2Cl
A)CH3CH2OH
B)CH3CH2CH2CH3
C)CH3OCH3
D)CH3CH2CH3
E)CH3CH2CH2Cl

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following compounds is expected to have the lowest vapor pressure?
A)CH3OCH3
B)CH3CH2F
C)CH3CH2OH
D)CH3CH2CH2CH3
E)CH3CH2CH3
A)CH3OCH3
B)CH3CH2F
C)CH3CH2OH
D)CH3CH2CH2CH3
E)CH3CH2CH3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following compounds has the lowest normal boiling point?
A)CH3CH2CH2NH2
B)CH3CH2CH2F
C)CH3CH2CH2OH
D)CH3CH2COOH
E)CH3CH(OH)CH3
A)CH3CH2CH2NH2
B)CH3CH2CH2F
C)CH3CH2CH2OH
D)CH3CH2COOH
E)CH3CH(OH)CH3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
58
Rank the following in order of increasing normal boiling point: N2,O2,Br2,Xe.
A)N2 < O2 < Xe < Br2
B)O2 < N2 < Xe < Br2
C)Br2 < Xe < N2 < O2
D)N2 < O2 < Br2 < Xe
E)Xe < Br2 < N2 < O2
A)N2 < O2 < Xe < Br2
B)O2 < N2 < Xe < Br2
C)Br2 < Xe < N2 < O2
D)N2 < O2 < Br2 < Xe
E)Xe < Br2 < N2 < O2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
59
Which one of the following decreases as the strength of the attractive intermolecular forces increases?
A)The normal boiling temperature.
B)The vapor pressure of a liquid.
C)The extent of deviations from the ideal gas law.
D)The heat of vaporization.
E)The sublimation temperature of a solid.
A)The normal boiling temperature.
B)The vapor pressure of a liquid.
C)The extent of deviations from the ideal gas law.
D)The heat of vaporization.
E)The sublimation temperature of a solid.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following pure substances has the lowest vapor pressure at 25°C?
A)SbH3
B)NH3
C)PH3
D)AsH3
E)H2O
A)SbH3
B)NH3
C)PH3
D)AsH3
E)H2O

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
61
What is the maximum number of hydrogen bonds in which a water molecule could participate?
A)2
B)4
C)3
D)6
E)5
A)2
B)4
C)3
D)6
E)5

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following is not a covalent network solid?
A)diamond
B)silicon carbide
C)quartz
D)iron
E)graphite
A)diamond
B)silicon carbide
C)quartz
D)iron
E)graphite

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following pure substances may exhibit hydrogen bonding?
A)CH3Cl
B)CH3OCH3
C)H2CO
D)N(CH3)3
E)H2NNH2
A)CH3Cl
B)CH3OCH3
C)H2CO
D)N(CH3)3
E)H2NNH2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following pure substances has the highest normal melting point?
A)KF
B)KI
C)NaF
D)NaCl
E)NaI
A)KF
B)KI
C)NaF
D)NaCl
E)NaI

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
65
Which pure substance exhibits hydrogen bonding?
A)HNF2
B)B2H6
C)HBr
D)H2S
E)CaH2
A)HNF2
B)B2H6
C)HBr
D)H2S
E)CaH2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
66
The strongest intermolecular forces present in a sample of pure I2 are
A)London forces.
B)dipole-dipole forces.
C)metallic bonds.
D)covalent network bonds.
E)covalent bonds.
A)London forces.
B)dipole-dipole forces.
C)metallic bonds.
D)covalent network bonds.
E)covalent bonds.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
67
A solid has a very high melting point,is hard,and in the molten state is a non-conductor.The solid is most likely
A)a covalent network solid.
B)a metallic solid.
C)an amorphous solid.
D)a molecular solid.
E)an ionic solid.
A)a covalent network solid.
B)a metallic solid.
C)an amorphous solid.
D)a molecular solid.
E)an ionic solid.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
68
Which bonding interaction best describes the strongest intermolecular forces in AlH3?
A)dipole-dipole forces
B)metallic bonding
C)ionic bonding
D)London dispersion forces
E)primarily hydrogen bonding
A)dipole-dipole forces
B)metallic bonding
C)ionic bonding
D)London dispersion forces
E)primarily hydrogen bonding

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following best describes silane (SiH4)at room temperature and pressure?
A)ionic solid
B)covalent network solid
C)nonpolar molecular gas
D)polar molecular gas
E)metallic solid
A)ionic solid
B)covalent network solid
C)nonpolar molecular gas
D)polar molecular gas
E)metallic solid

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following is a molecular solid?
A)NaCl
B)CH4
C)SiO2
D)C(graphite)
E)C(diamond)
A)NaCl
B)CH4
C)SiO2
D)C(graphite)
E)C(diamond)

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following is an ionic solid?
A)SiO2(s)
B)Ne(s)
C)Na(s)
D)CsF(s)
E)CO2(s)
A)SiO2(s)
B)Ne(s)
C)Na(s)
D)CsF(s)
E)CO2(s)

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
72
Van der Waals forces must be broken to melt this type of solid.
A)A covalent network solid.
B)A metallic solid.
C)A molecular solid.
D)An ionic solid.
E)none of the above
A)A covalent network solid.
B)A metallic solid.
C)A molecular solid.
D)An ionic solid.
E)none of the above

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following is a molecular solid?
A)carborundum,SiC
B)quartz
C)glass
D)hydrogen chloride
E)potassium
A)carborundum,SiC
B)quartz
C)glass
D)hydrogen chloride
E)potassium

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
74
The strongest intermolecular forces between molecules of PH3 are
A)hydrogen bonds.
B)covalent bonds.
C)ionic bonds.
D)London forces.
E)dipole-dipole attractions.
A)hydrogen bonds.
B)covalent bonds.
C)ionic bonds.
D)London forces.
E)dipole-dipole attractions.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
75
Which substance can be described as cations bonded together by mobile electrons?
A)S8(s)
B)Ag(s)
C)HCl(l)
D)KCl(s)
E)Kr(l)
A)S8(s)
B)Ag(s)
C)HCl(l)
D)KCl(s)
E)Kr(l)

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following best describes carbon dioxide (CO2)at room temperature and pressure?
A)ionic solid
B)nonpolar molecular gas
C)metallic solid
D)polar molecular gas
E)covalent network solid
A)ionic solid
B)nonpolar molecular gas
C)metallic solid
D)polar molecular gas
E)covalent network solid

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following is the strongest intermolecular force present in dry ice,CO2(s)?
A)covalent bonding
B)hydrogen bonding
C)ionic bonding
D)London forces
E)metallic bonding
A)covalent bonding
B)hydrogen bonding
C)ionic bonding
D)London forces
E)metallic bonding

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following best describes calcium hydride (CaH2)at room temperature and pressure?
A)nonpolar molecular gas
B)metallic solid
C)ionic solid
D)polar molecular gas
E)covalent network solid
A)nonpolar molecular gas
B)metallic solid
C)ionic solid
D)polar molecular gas
E)covalent network solid

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
79
The molecules in a sample of solid SO2 are attracted to each other by a combination of
A)H-bonding and ionic bonding.
B)covalent bonding and dipole-dipole interactions.
C)London forces and H-bonding.
D)London forces and dipole-dipole interactions.
E)none of these
A)H-bonding and ionic bonding.
B)covalent bonding and dipole-dipole interactions.
C)London forces and H-bonding.
D)London forces and dipole-dipole interactions.
E)none of these

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following substances has the weakest intermolecular forces?
A)I2
B)C8H18
C)SiH4
D)CH3CH2CH2CH2OH
E)SbCl3
A)I2
B)C8H18
C)SiH4
D)CH3CH2CH2CH2OH
E)SbCl3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck


