Exam 11: States of Matter; Liquids and Solids
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
From a consideration of the phase diagram below,a change from point M to point N corresponds to 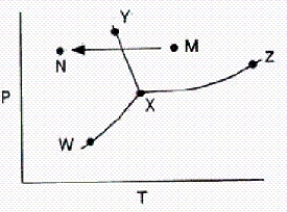
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
E
What is the maximum number of hydrogen bonds in which a water molecule could participate?
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
B
Knowing that ΔHvap for water is 40.7 kJ/mol,calculate Pvap of water at 37°C.
Free
(Multiple Choice)
4.8/5  (25)
(25)
Correct Answer:
A
Sulfur trioxide (SO3)is able to be liquefied at low temperatures due to which intermolecular force?
(Multiple Choice)
4.9/5  (34)
(34)
What is the enthalpy of vaporization of a compound that has a vapor pressure of 151 mmHg at 247 K and 1.52 mmHg at 187 K? (R = 8.31 J/(K· mol))
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following pure substances has the lowest melting point?
(Multiple Choice)
4.8/5  (37)
(37)
Lithium chloride crystallizes in a face-centered cubic structure.The unit cell length is 5.14 × 10-8 cm.The chloride ions are touching each other along the face diagonal of the unit cell.The lithium ions fit into the holes between the chloride ions.What is the mass of Li Cl in a unit cell?
(Multiple Choice)
4.8/5  (30)
(30)
How many atoms are there in a body-centered cubic unit cell of rubidium?
(Multiple Choice)
5.0/5  (32)
(32)
Which of the following pure substances has the highest melting point?
(Multiple Choice)
4.9/5  (43)
(43)
A low melting solid readily dissolves in water to give a nonconducting solution.The solid is most likely a
(Multiple Choice)
4.9/5  (35)
(35)
Below is a phase diagram for a substance. 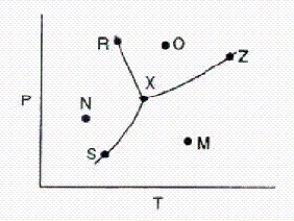 Which line represents the vapor-pressure curve of the substance?
Which line represents the vapor-pressure curve of the substance?
(Multiple Choice)
4.8/5  (30)
(30)
In which of the following substances are intermolecular forces of attraction absent?
(Multiple Choice)
4.8/5  (40)
(40)
In any cubic lattice an atom lying at the corner of a unit cell is shared equally by how many unit cells?
(Multiple Choice)
4.8/5  (35)
(35)
Below is a phase diagram for a substance. 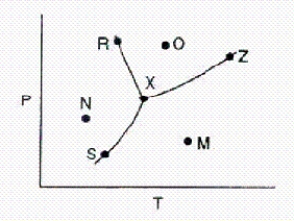 What is the name for point X on the diagram?
What is the name for point X on the diagram?
(Multiple Choice)
4.9/5  (33)
(33)
What is the name for the following phase change?
I2(l)→ I2(g)
(Multiple Choice)
4.7/5  (36)
(36)
In a certain mountain range,water boils at 94°C.What is the atmospheric pressure under these conditions? The enthalpy of vaporization of water at 100°C is 40.7 kJ/mol.(R = 8.31 J/(K · mol))
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following pure substances has the highest vapor pressure at room temperature?
(Multiple Choice)
4.9/5  (34)
(34)
Showing 1 - 20 of 123
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)