Deck 12: Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/119
Play
Full screen (f)
Deck 12: Solutions
1
In general,which of the following type(s)of solid(s)would exhibit the greatest solubility in a polar solvent?
A)network covalent
B)ionic or polar molecular
C)ionic
D)metallic
E)polar molecular
A)network covalent
B)ionic or polar molecular
C)ionic
D)metallic
E)polar molecular
ionic or polar molecular
2
Which of the following gases is least soluble in water?
A)CO2
B)SO3
C)NH3
D)N2
E)HCl
A)CO2
B)SO3
C)NH3
D)N2
E)HCl
N2
3
Henry's Law constant is 0.034 mol/kg⋅bar and 0.0013 mol/kg⋅bar for CO2 and O2 respectively at 25°C.What pressure of O2 is required to achieve the same solubility as 0.368 bar of CO2?
A)9.6 bar
B)0.0 bar
C)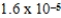 bar
bar
D)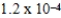 bar
bar
E)0.1 bar
A)9.6 bar
B)0.0 bar
C)
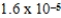 bar
barD)
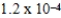 bar
barE)0.1 bar
9.6 bar
4
Which of the following favor(s)the solubility of an ionic solid in a liquid solvent?
A)a small magnitude of the lattice energy of the solute
B)a large magnitude of the solvation energy of the ions
C)a large polarity of the solvent
D)all of the above
E)none of the above
A)a small magnitude of the lattice energy of the solute
B)a large magnitude of the solvation energy of the ions
C)a large polarity of the solvent
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
5
The solubility of 1-pentanol in water is 2.7 g per 100 g of water at 25°C.What is the maximum amount of 1-pentanol that will dissolve in 4.1g of water at 25°C?
A)0.11 g
B)0.65 g
C)2.7 g
D)11 g
E)0.0065 g
A)0.11 g
B)0.65 g
C)2.7 g
D)11 g
E)0.0065 g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is a correct statement of Henry's law?
A)The concentration of a gas in solution is directly proportional to the mole fraction of solvent.
B)The concentration of a gas in solution is inversely proportional to temperature.
C)The concentration of a gas in solution is independent of pressure.
D)The concentration of a gas in a solution is inversely proportional to pressure.
E)none of these
A)The concentration of a gas in solution is directly proportional to the mole fraction of solvent.
B)The concentration of a gas in solution is inversely proportional to temperature.
C)The concentration of a gas in solution is independent of pressure.
D)The concentration of a gas in a solution is inversely proportional to pressure.
E)none of these

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following statements best describes what happens when a small amount of solid rubidium chloride is dissolved in water?
A)The heat from the warm water melts the solid,making it a liquid.
B)Nothing happens,because rubidium chloride is insoluble in water.
C)The solid RbCl breaks apart into separate Rb and Cl atoms by interacting with the water molecules.
D)The water molecules surround each ion in the solid RbCl,separating the Rb ions from the Cl ions.
E)The solid undergoes a chemical change by reacting with the water.
A)The heat from the warm water melts the solid,making it a liquid.
B)Nothing happens,because rubidium chloride is insoluble in water.
C)The solid RbCl breaks apart into separate Rb and Cl atoms by interacting with the water molecules.
D)The water molecules surround each ion in the solid RbCl,separating the Rb ions from the Cl ions.
E)The solid undergoes a chemical change by reacting with the water.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following compounds is least soluble in water?
A)CH3CH2CH2NH2
B)CH3CH2CH2F
C)CH3CH(OH)CH3
D)CH3CH2COOH
E)CH3CH2NHCH3
A)CH3CH2CH2NH2
B)CH3CH2CH2F
C)CH3CH(OH)CH3
D)CH3CH2COOH
E)CH3CH2NHCH3

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
9
According to the National Institute of Standards webbook,the Henry's Law constant for N2 gas is 0.000639 mol/kg⋅bar at 25°C What is the Henry's law constant in units of mol/kg⋅atm? (1 bar = 0.9869 atm; 1 atm = 760 mmHg)
A)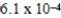 × 10-4 mol/kg⋅atm
× 10-4 mol/kg⋅atm
B)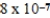 × 10-7 mol/kg⋅atm
× 10-7 mol/kg⋅atm
C)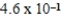 × 10-1 mol/kg⋅atm
× 10-1 mol/kg⋅atm
D)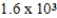 × 103 mol/kg⋅atm
× 103 mol/kg⋅atm
E)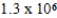 × 106 mol/kg⋅atm
× 106 mol/kg⋅atm
A)
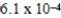 × 10-4 mol/kg⋅atm
× 10-4 mol/kg⋅atmB)
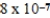 × 10-7 mol/kg⋅atm
× 10-7 mol/kg⋅atmC)
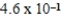 × 10-1 mol/kg⋅atm
× 10-1 mol/kg⋅atmD)
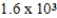 × 103 mol/kg⋅atm
× 103 mol/kg⋅atmE)
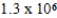 × 106 mol/kg⋅atm
× 106 mol/kg⋅atm
Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
10
Consider the following gas-aqueous liquid equilibrium for a closed system at a constant temperature.
O2(g)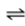 O2(aq)
O2(aq)
What is the effect on the equilibrium composition of the liquid when the partial pressure of O2 gas above the liquid is decreased?
A)The amount of O2 dissolved in the liquid decreases.
B)The amount of O2 dissolved in the liquid increases.
C)The amount of O2 dissolved in the liquid does not change.
D)Not enough information is provided to answer the question.
E)Either A or B.
O2(g)
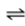 O2(aq)
O2(aq)What is the effect on the equilibrium composition of the liquid when the partial pressure of O2 gas above the liquid is decreased?
A)The amount of O2 dissolved in the liquid decreases.
B)The amount of O2 dissolved in the liquid increases.
C)The amount of O2 dissolved in the liquid does not change.
D)Not enough information is provided to answer the question.
E)Either A or B.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
11
Consider the following gas-liquid equilibrium for an aqueous system at a constant partial pressure of N2.
N2(g)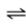
N2(aq)
What is the effect on the equilibrium composition of the liquid when the temperature of the liquid is increased?
A)The amount of N2 dissolved in the liquid increases.
B)The amount of N2 dissolved in the liquid decreases.
C)The amount of N2 dissolved in the liquid does not change.
D)Not enough information is provided to answer the question.
E)Either A or B could occur.
N2(g)
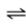
N2(aq)
What is the effect on the equilibrium composition of the liquid when the temperature of the liquid is increased?
A)The amount of N2 dissolved in the liquid increases.
B)The amount of N2 dissolved in the liquid decreases.
C)The amount of N2 dissolved in the liquid does not change.
D)Not enough information is provided to answer the question.
E)Either A or B could occur.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following correctly states the relationship between the solubility of a substance in water and temperature?
A)The solubility of a substance in water increases as the temperature rises,especially for gases.
B)The solubility of a substance in water decreases as the temperature lowers,especially for gases.
C)The relationship between the solubility of a substance in water and temperature cannot be accurately predicted,especially for ionic solids.
D)The solubility of a substance in water decreases as the temperature rises,especially for ionic solids.
E)Two of these are correct.
A)The solubility of a substance in water increases as the temperature rises,especially for gases.
B)The solubility of a substance in water decreases as the temperature lowers,especially for gases.
C)The relationship between the solubility of a substance in water and temperature cannot be accurately predicted,especially for ionic solids.
D)The solubility of a substance in water decreases as the temperature rises,especially for ionic solids.
E)Two of these are correct.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following sets of conditions favors maximum solubility of an ionic solute in water?
A)The enthalpy of hydration of the cation should be equal to the enthalpy of hydration of the anion,regardless of the magnitude of the lattice energy.
B)The magnitude of the lattice energy should be small,and the enthalpy of hydration of the ions should be large.
C)The magnitude of the lattice energy should be small,and the enthalpy of hydration of the ions should be small.
D)The magnitude of the lattice energy should be large,and the enthalpy of hydration of the ions should be small.
E)The magnitude of the lattice energy should be large,and the enthalpy of hydration of the ions should be large.
A)The enthalpy of hydration of the cation should be equal to the enthalpy of hydration of the anion,regardless of the magnitude of the lattice energy.
B)The magnitude of the lattice energy should be small,and the enthalpy of hydration of the ions should be large.
C)The magnitude of the lattice energy should be small,and the enthalpy of hydration of the ions should be small.
D)The magnitude of the lattice energy should be large,and the enthalpy of hydration of the ions should be small.
E)The magnitude of the lattice energy should be large,and the enthalpy of hydration of the ions should be large.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
14
At a particular temperature the solubility of O2 in water is 0.590 g/L at an oxygen pressure of around 15.2 atm.What is the Henry's law constant for O2 (in units of L · atm/mol)?
A)3.88 × 10-2
B)8.24 × 102
C)2.80× 10-1
D)1.21× 10-3
E)None of the above are within 5% of the correct answer.
A)3.88 × 10-2
B)8.24 × 102
C)2.80× 10-1
D)1.21× 10-3
E)None of the above are within 5% of the correct answer.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following pure liquids is the best solvent for sodium fluoride?
A)CCl4(l)
B)C2Cl6(l)
C)HCl(l)
D)BCl3(l)
E)PCl5(l)
A)CCl4(l)
B)C2Cl6(l)
C)HCl(l)
D)BCl3(l)
E)PCl5(l)

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following sets of conditions favors maximum solubility of solute in solvent?
A)The intermolecular forces between solute and solvent molecules are much weaker than the intermolecular forces between solute molecules,but much stronger than the intermolecular forces between solvent molecules.
B)The intermolecular forces between solute and solvent molecules are much stronger than the intermolecular forces between solute molecules or the intermolecular forces between solvent molecules.
C)The intermolecular forces between solute and solvent molecules are much stronger than the intermolecular forces between solvent molecules,but much weaker than the intermolecular forces between solute molecules.
D)The intermolecular forces between solute and solvent molecules are much stronger than the intermolecular forces between solute molecules,but much weaker than the intermolecular forces between solvent molecules.
E)The intermolecular forces between solute and solvent molecules are much weaker than the intermolecular forces between solute molecules or the intermolecular forces between solvent molecules.
A)The intermolecular forces between solute and solvent molecules are much weaker than the intermolecular forces between solute molecules,but much stronger than the intermolecular forces between solvent molecules.
B)The intermolecular forces between solute and solvent molecules are much stronger than the intermolecular forces between solute molecules or the intermolecular forces between solvent molecules.
C)The intermolecular forces between solute and solvent molecules are much stronger than the intermolecular forces between solvent molecules,but much weaker than the intermolecular forces between solute molecules.
D)The intermolecular forces between solute and solvent molecules are much stronger than the intermolecular forces between solute molecules,but much weaker than the intermolecular forces between solvent molecules.
E)The intermolecular forces between solute and solvent molecules are much weaker than the intermolecular forces between solute molecules or the intermolecular forces between solvent molecules.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following pure liquids is the best solvent for carbon disulfide?
A)C6H6(l)
B)NH3(l)
C)CH3OH(l)
D)H2O(l)
E)HBr(l)
A)C6H6(l)
B)NH3(l)
C)CH3OH(l)
D)H2O(l)
E)HBr(l)

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
18
The solubility of a gas in a liquid can always be increased by
A)decreasing the pressure of the gas above the solvent.
B)increasing the pressure of the gas above the solvent.
C)increasing the temperature of the solvent.
D)decreasing the polarity of the solvent.
E)decreasing the temperature of the gas above the solvent.
A)decreasing the pressure of the gas above the solvent.
B)increasing the pressure of the gas above the solvent.
C)increasing the temperature of the solvent.
D)decreasing the polarity of the solvent.
E)decreasing the temperature of the gas above the solvent.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
19
The dissolution of ionic compounds in water depends only on the hydration energy of the ions.
A)1 only
B)2 only
C)3 only
D)1 and 2
E)1,2,and 3
A)1 only
B)2 only
C)3 only
D)1 and 2
E)1,2,and 3

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
20
How does the solubility of a gas in a solvent depend on pressure and temperature?
A)Increasing the partial pressure of the gas while increasing the temperature increases the solubility of the gas.
B)Decreasing the partial pressure of the gas while decreasing the temperature increases the solubility of the gas.
C)Increasing the partial pressure of the gas while decreasing the temperature increases the solubility of the gas.
D)Decreasing the partial pressure of the gas while increasing the temperature increases the solubility of the gas.
E)Gas solubility is unaffected by pressure or temperature.
A)Increasing the partial pressure of the gas while increasing the temperature increases the solubility of the gas.
B)Decreasing the partial pressure of the gas while decreasing the temperature increases the solubility of the gas.
C)Increasing the partial pressure of the gas while decreasing the temperature increases the solubility of the gas.
D)Decreasing the partial pressure of the gas while increasing the temperature increases the solubility of the gas.
E)Gas solubility is unaffected by pressure or temperature.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
21
What is the mass percent of an aqueous sodium hydroxide solution in which the molality of NaOH is 10.7 m? The density of the solution is 1.3311 g/mL.
A)0.1%
B)68.9%
C)1.7%
D)30.0%
E)0.3%
A)0.1%
B)68.9%
C)1.7%
D)30.0%
E)0.3%

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
22
The molarity of a solution is defined as the
A)moles of solute per liter of solvent.
B)grams of solute per kilogram of solvent.
C)grams of solute per liter of solution.
D)moles of solute per liter of solution.
E)moles of solute per kilogram of solvent.
A)moles of solute per liter of solvent.
B)grams of solute per kilogram of solvent.
C)grams of solute per liter of solution.
D)moles of solute per liter of solution.
E)moles of solute per kilogram of solvent.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
23
If the solubility of O2 at 0.160 bar and 25°C is 6.65 g/100 g H2O,what is the solubility of O2 at a pressure of 1.84 bar and 25°C?
A)77 g/100 g H2O
B)22.5 g/100 g H2O
C)1.7 g/100 g H2O
D)0.0 g/100 g H2O
E)0.6 g/100 g H2O
A)77 g/100 g H2O
B)22.5 g/100 g H2O
C)1.7 g/100 g H2O
D)0.0 g/100 g H2O
E)0.6 g/100 g H2O

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
24
What mass of an aqueous 33.7% sodium chloride solution contains 88.1 g of water?
A)132 g
B)44.7 g
C)11.9 g
D)29.6 g
E)88.1 g
A)132 g
B)44.7 g
C)11.9 g
D)29.6 g
E)88.1 g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
25
The volume of a 34.6% (by mass)solution is 129.0 mL.The density of the solution is 1.116 g/mL.What is the mass of solute in this solution?
A)144 g
B)49.8 g
C)416 g
D)40.0 g
E)94 g
A)144 g
B)49.8 g
C)416 g
D)40.0 g
E)94 g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
26
What is the molarity of a 10.0% by mass hydrochloric acid solution? The density of the solution is 1.0474 g/mL.
A)7 M
B)0 M
C)9 M
D)3 M
E)1 M
A)7 M
B)0 M
C)9 M
D)3 M
E)1 M

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
27
A concentrated acetic acid solution has a density of 1.05 g/mL at 25°C and is 17.4 M.What is the percent by mass of CH3COOH in the solution?
A)99.5% CH3COOH by mass
B)1.7% CH3COOH by mass
C)0.6% CH3COOH by mass
D)2.8% CH3COOH by mass
E)57.1% CH3COOH by mass
A)99.5% CH3COOH by mass
B)1.7% CH3COOH by mass
C)0.6% CH3COOH by mass
D)2.8% CH3COOH by mass
E)57.1% CH3COOH by mass

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
28
What is the mass percent of an aqueous sodium hydroxide solution in which the mole fraction of NaOH is 0.1010? The density of the solution is 1.2219 g/mL.
A)20.0%
B)12.0%
C)68.9%
D)12.3%
E)3.3%
A)20.0%
B)12.0%
C)68.9%
D)12.3%
E)3.3%

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
29
A concentrated sulfuric acid solution is 65.0% H2SO4 by mass and has a density of 1.55 g/mL at 20°C.What is the mass of 9.00 L of the concentrated sulfuric acid solution?
A)5.85 kg
B)9.00 kg
C)21.4 kg
D)3.77 kg
E)13.9 kg
A)5.85 kg
B)9.00 kg
C)21.4 kg
D)3.77 kg
E)13.9 kg

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is not a colligative property?
A)boiling-point elevation
B)osmotic pressure
C)lattice energy
D)freezing-point lowering
E)vapor-pressure lowering
A)boiling-point elevation
B)osmotic pressure
C)lattice energy
D)freezing-point lowering
E)vapor-pressure lowering

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
31
The molarity of a solution is defined as the
A)moles of solute per liter of solvent.
B)moles of solute per kilogram of solution.
C)moles of solute per mole of solution.
D)moles of solute per kilogram of solvent.
E)moles of solute per liter of solution.
A)moles of solute per liter of solvent.
B)moles of solute per kilogram of solution.
C)moles of solute per mole of solution.
D)moles of solute per kilogram of solvent.
E)moles of solute per liter of solution.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
32
The molality of a solution is defined as
A)moles of solute per liter of solution.
B)the gram molecular weight of solute per kilogram of solvent.
C)moles of solute per kilogram of solvent.
D)grams of solute per liter of solution.
E)moles of solute per kilogram of solution.
A)moles of solute per liter of solution.
B)the gram molecular weight of solute per kilogram of solvent.
C)moles of solute per kilogram of solvent.
D)grams of solute per liter of solution.
E)moles of solute per kilogram of solution.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
33
What is the mass of H2SO4 in a 38.2-cm3 sample of concentrated sulfuric acid that has a density of 1.84 g/cm3 and consists of 98.3% H2SO4?
A)37.6 g
B)69.1 g
C)4.73 g
D)1.81 g
E)20.4 g
A)37.6 g
B)69.1 g
C)4.73 g
D)1.81 g
E)20.4 g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
34
What is the percent Na2CO3 by mass in a 1.92 molal aqueous solution?
A)0.160%
B)16.9%
C)99.5%
D)20.4%
E)19.2%
A)0.160%
B)16.9%
C)99.5%
D)20.4%
E)19.2%

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
35
What is the mass percent of an aqueous sodium hydroxide solution in which the molarity of NaOH is 9.98 M? The density of the solution is 1.33 g/mL.
A)0.1%
B)30.0%
C)13.2%
D)1.7%
E)68.9%
A)0.1%
B)30.0%
C)13.2%
D)1.7%
E)68.9%

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
36
As the number of solute particles in a given volume of solution increases,
A)the boiling point will increase and the vapor pressure will increase.
B)the freezing point will decrease and the vapor pressure will decrease.
C)the freezing point will increase and the vapor pressure will increase.
D)the boiling point will decrease and the vapor pressure will decrease.
E)the osmotic pressure will decrease and the lattice energy will increase.
A)the boiling point will increase and the vapor pressure will increase.
B)the freezing point will decrease and the vapor pressure will decrease.
C)the freezing point will increase and the vapor pressure will increase.
D)the boiling point will decrease and the vapor pressure will decrease.
E)the osmotic pressure will decrease and the lattice energy will increase.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
37
The volume of a 24.0% (by mass)solution is 65.2 mL.The density of the solution is 1.072 g/mL.What is the mass of the solution?
A)69.8 g
B)61 g
C)1670 g
D)167 g
E)17 g
A)69.8 g
B)61 g
C)1670 g
D)167 g
E)17 g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
38
What mass of a 29.0% by mass glucose,C6H12O6,solution contains 60.0 g of glucose?
A)52.2 g
B)207 g
C)17.4 g
D)96.7 g
E)60.0 g
A)52.2 g
B)207 g
C)17.4 g
D)96.7 g
E)60.0 g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
39
A concentrated hydrofluoric acid solution is 49.0% HF by mass and has a density of 1.3406 g/mL at 25°C.What is the mass of HF per L of solution?
A)637 g HF /L soln
B)0.0013 g HF /L soln
C)376 g HF /L soln
D)0.265 g HF /L soln
E)3.76 g HF /L soln
A)637 g HF /L soln
B)0.0013 g HF /L soln
C)376 g HF /L soln
D)0.265 g HF /L soln
E)3.76 g HF /L soln

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
40
A concentrated sodium hydroxide solution is 50.5% NaOH by mass and has a density of 1.53 g/mL at 25°C.What is the molarity of NaOH?
A)19.3 M
B)0.0612 M
C)8.25 M
D)12.1 M
E)0.0825 M
A)19.3 M
B)0.0612 M
C)8.25 M
D)12.1 M
E)0.0825 M

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
41
How many moles of urea (60.g/mol)must be dissolved in 66.6g of water to give a 2.4 m solution?
A)1.4 × 102 mol
B)2.4 mol
C)0.0024 mol
D)0.15 mol
E)9.0 × 102 mol
A)1.4 × 102 mol
B)2.4 mol
C)0.0024 mol
D)0.15 mol
E)9.0 × 102 mol

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
42
A concentrated ammonia solution is 28.0% NH3 by mass and has a density of 0.939 g/mL at 25°C.The remainder of material is solvent.What is the molality of NH3 in the solution?
A)22.8 m
B)16.4 m
C)12.8 m
D)437 m
E)0.236 m
A)22.8 m
B)16.4 m
C)12.8 m
D)437 m
E)0.236 m

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
43
What mass of a solution labeled 6.3% sucrose (C12H22O11,342 g/mol)by mass contains 11.0 g of sucrose?
A)2.7 g
B)21 g
C)170 g
D)0 g
E)69 g
A)2.7 g
B)21 g
C)170 g
D)0 g
E)69 g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
44
What is the molality of ethanol,C2H5OH,in an aqueous solution that is 36.4% ethanol by mass?
A)1 m
B)0 m
C)12 m
D)19 m
E)97 m
A)1 m
B)0 m
C)12 m
D)19 m
E)97 m

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
45
What is the mole fraction of urea in a solution that contains 2.7 mol of urea and 4.6 mol of water?
A)0.77
B)0.37
C)0.47
D)0.57
E)0.63
A)0.77
B)0.37
C)0.47
D)0.57
E)0.63

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
46
What is the mole fraction of urea,CO(NH2)2,in a solution prepared by dissolving 4.0 g of urea in 32.0 g of methanol,CH3OH?
A)0.89
B)0.11
C)0.063
D)0.94
E)0.19
A)0.89
B)0.11
C)0.063
D)0.94
E)0.19

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
47
What is the molality of a solution that contains 9.16 g of glucose,C6H12O6,in 228.5 g of water?
A)0.223 m
B)0.0509 m
C)0.00399 m
D)0.0116 m
E)0.0401 m
A)0.223 m
B)0.0509 m
C)0.00399 m
D)0.0116 m
E)0.0401 m

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
48
What is the mole fraction of water in a solution that contains 8.0 mol of ethanol (C2H5OH)and 1.6 mol of water?
A)0.17
B)0.08
C)0.50
D)0.20
E)0.83
A)0.17
B)0.08
C)0.50
D)0.20
E)0.83

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
49
What is the mole fraction of urea,CH4N2O,in an aqueous solution that is 37% urea by mass?
A)0.15
B)0.85
C)0.37
D)0.54
E)0.66
A)0.15
B)0.85
C)0.37
D)0.54
E)0.66

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
50
If 11.2 g of naphthalene,C10H8,is dissolved in 107.8 g of chloroform,CHCl3,what is the molality of the solution?
A)0.0875 m
B)12.4 m
C)0.811 m
D)0.0941 m
E)0.0969 m
A)0.0875 m
B)12.4 m
C)0.811 m
D)0.0941 m
E)0.0969 m

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
51
What is the mole fraction of toluene in a solution of 2.7 mol of benzene and 5.4 mol of toluene?
A)0.70
B)0.54
C)0.67
D)0.24
E)0.33
A)0.70
B)0.54
C)0.67
D)0.24
E)0.33

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
52
What is the mole fraction of water in a water-ethanol solution that is 47.0% water by mass? (Ethanol is C2H5OH.)
A)0.28
B)0.31
C)0.26
D)0.69
E)0.53
A)0.28
B)0.31
C)0.26
D)0.69
E)0.53

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
53
What is the molality of a solution prepared by dissolving 0.114 mol of chloroform,CHCl3,in 476 g of toluene,C6H5CH3?
A)0.0287 m
B)0.0216 m
C)0.239 m
D)0.0220 m
E)0.543 m
A)0.0287 m
B)0.0216 m
C)0.239 m
D)0.0220 m
E)0.543 m

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
54
What is the molality of a solution that contains 74.2 g of 1,4-dichlorobenzene (C6H4Cl2)in 492 mL of carbon tetrachloride (CCl4)? The density of CCl4 is 1.60 g/mL.
A)0.158 m
B)0.642 m
C)0.151 m
D)0.094 m
E)1.64 m
A)0.158 m
B)0.642 m
C)0.151 m
D)0.094 m
E)1.64 m

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
55
For a solution containing only one solute dissolved in a solvent,we can calculate the mole fraction of the solvent directly,given only
A)the molar mass of the solute.
B)the density of the solution.
C)the molar mass of the solvent.
D)the mole fraction of the solute.
E)the molarity of the solution.
A)the molar mass of the solute.
B)the density of the solution.
C)the molar mass of the solvent.
D)the mole fraction of the solute.
E)the molarity of the solution.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
56
What is the molality of a solution prepared by dissolving 9.01 g of urea,NH2CONH2,in 93.8 g of water?
A)0.0876 m
B)0.096498 m
C)0.028985 m
D)1.59 m
E)0.00145 m
A)0.0876 m
B)0.096498 m
C)0.028985 m
D)1.59 m
E)0.00145 m

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
57
What is the molality of a 30.0% by mass hydrochloric acid solution? The density of the solution is 1.1493 g/mL.
A)0.0344 m
B)11.7 m
C)0.377 m
D)9.29 m
E)9.45 m
A)0.0344 m
B)11.7 m
C)0.377 m
D)9.29 m
E)9.45 m

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
58
Which way of expressing concentration is used to relate the vapor pressure of a solution to the amount of nonvolatile solute dissolved in the solution?
A)mole fraction
B)molarity
C)osmotic pressure
D)mass percent
E)molality
A)mole fraction
B)molarity
C)osmotic pressure
D)mass percent
E)molality

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
59
A 3.140 molal solution of NaCl is prepared.How many grams of NaCl are present in a sample containing 2.692 kg of water?
A)299.7 g
B)494.0 g
C)144.6 g
D)845.3 g
E)none of these
A)299.7 g
B)494.0 g
C)144.6 g
D)845.3 g
E)none of these

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
60
A 12.0% sucrose solution by mass has a density of 1.05 g/cm3.What mass of sucrose is present in a 29.0-mL sample of this solution?
A)3.65 g
B)3.31 g
C)0.126 g
D)253 g
E)3.48 g
A)3.65 g
B)3.31 g
C)0.126 g
D)253 g
E)3.48 g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
61
If a 23.6-g sample of a nonelectrolyte is dissolved in 136.0 g of water,the resulting solution will freeze at -0.85°C.What is the molar mass of the nonelectrolyte? (Kf for water is 1.858°C/m.)
A)78 g/mol
B)0.38 g/mol
C)870 g/mol
D)270 g/mol
E)370 g/mol
A)78 g/mol
B)0.38 g/mol
C)870 g/mol
D)270 g/mol
E)370 g/mol

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
62
Trimellitic acid is an organic acid that has a composition of 51.44% C,2.88% H,and 45.68% O by mass.A 6.96-g sample of trimellitic acid dissolved in 30.00 g of acetone,CH3COCH3,has a boiling point of 58.09°C.What is the molecular formula of trimellitic acid? (Kb for acetone is 1.71°C/m,and pure acetone has a boiling point of 56.20°C.)
A)CH2O
B)C9H6O6
C)C3HO2
D)C18HO16
E)C6H2O4
A)CH2O
B)C9H6O6
C)C3HO2
D)C18HO16
E)C6H2O4

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
63
A liquid-liquid solution is called an ideal solution if
I.it obeys PV = nRT.
II.it obeys Raoult's law.
III.solute-solute,solvent-solvent,and solute-solvent interactions are very similar.
IV.solute-solute,solvent-solvent,and solute-solvent interactions are quite different.
A)I,III,IV
B)I,II,III
C)II,III only
D)I,II,IV
E)II,IV only
I.it obeys PV = nRT.
II.it obeys Raoult's law.
III.solute-solute,solvent-solvent,and solute-solvent interactions are very similar.
IV.solute-solute,solvent-solvent,and solute-solvent interactions are quite different.
A)I,III,IV
B)I,II,III
C)II,III only
D)I,II,IV
E)II,IV only

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
64
What is the mass percent of ethylene glycol (HOCH2CH2OH)in a solution of ethylene glycol in water that has a freezing point of -17.8°C? (Kf for water is 1.858°C/m.)
A)99.8%
B)37.2%
C)59.4%
D)64.7%
E)9.58%
A)99.8%
B)37.2%
C)59.4%
D)64.7%
E)9.58%

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
65
What is the vapor pressure at 75°C of an aqueous solution prepared by the addition of 58.4 g of the nonvolatile solute urea,CO(NH2)2,to 172 g of water? The vapor pressure of pure water at 75°C is 290.mmHg.
A)136 mmHg
B)26.8 mmHg
C)153 mmHg
D)263 mmHg
E)216 mmHg
A)136 mmHg
B)26.8 mmHg
C)153 mmHg
D)263 mmHg
E)216 mmHg

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
66
What is the molar mass of an aromatic hydrocarbon if 0.75 g of the compound depresses the freezing point of 123 g of benzene by 0.28°C? (Kf for benzene is 5.12°C/m.)
A)43 g/mol
B)150 g/mol
C)110.00 g/mol 110
D)2900 g/mol
E)140 g/mol
A)43 g/mol
B)150 g/mol
C)110.00 g/mol 110
D)2900 g/mol
E)140 g/mol

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
67
Thyroxine,an important hormone that controls the rate of metabolism in the body,can be isolated from the thyroid gland.If 0.454 g of thyroxine is dissolved in 10.0 g of benzene,the freezing point of the solution could be measured as 5.144°C.Pure benzene freezes at 5.444°C and has a value for the molal freezing-point-depression constant of Kf of 5.12°C/m.What is the approximate molar mass of thyroxine?
A)775 g/mol
B)7.75 g/mol
C)7.75 × 105 g/mol
D)42.7 g/mol
E)11.3 g/mol
A)775 g/mol
B)7.75 g/mol
C)7.75 × 105 g/mol
D)42.7 g/mol
E)11.3 g/mol

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
68
Substance A has a greater molar mass than substance B.If 50 g of substance A are dissolved in 250 g of water in one beaker,and 50 g of substance B are dissolved in 250 g of water in another beaker,then
A)the vapor pressure of solution A will be lower than the vapor pressure of solution B.
B)the solution of A will freeze at a lower temperature than the solution of B.
C)the two solutions will have the same vapor pressure.
D)the boiling point of solution A will be lower than the boiling point of solution B.
E)the solution of A will have a higher osmotic pressure than the solution of B.
A)the vapor pressure of solution A will be lower than the vapor pressure of solution B.
B)the solution of A will freeze at a lower temperature than the solution of B.
C)the two solutions will have the same vapor pressure.
D)the boiling point of solution A will be lower than the boiling point of solution B.
E)the solution of A will have a higher osmotic pressure than the solution of B.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
69
What is the freezing point of a 0.19 m solution of glucose,C6H12O6,in water? (Kf for water is 1.858°C/m.)
A)0.18°C
B)0.35°C
C)-0.35°C
D)-0.18°C
E)-0.71°C
A)0.18°C
B)0.35°C
C)-0.35°C
D)-0.18°C
E)-0.71°C

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
70
What is the vapor pressure at 20°C of an ideal solution prepared by the addition of 1.36 g of the nonvolatile solute urea,CO(NH2)2,to 24.1 g of methanol,CH3OH? The vapor pressure of pure methanol at 20°C is 89.0 mmHg.
A)2.6708 mmHg
B)80.4 mmHg
C)84.2 mmHg
D)86.3 mmHg
E)8.51 mmHg
A)2.6708 mmHg
B)80.4 mmHg
C)84.2 mmHg
D)86.3 mmHg
E)8.51 mmHg

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
71
What is the boiling-point change for a solution containing 0.723 mol of naphthalene (a nonvolatile,nonionizing compound)in 250.g of liquid benzene? (Kb = 2.53°C/m for benzene)
A)7.32 °C
B)3.50 °C
C)0.457 °C
D)0.87 °C
E)1.829 °C
A)7.32 °C
B)3.50 °C
C)0.457 °C
D)0.87 °C
E)1.829 °C

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
72
Benzene,C6H6,and toluene,C6H5CH3,form ideal solutions.At 35°C the vapor pressure of benzene is 160.torr and that of toluene is 50.0 torr.In an experiment,55.6 g of benzene and 86.6 g of toluene are placed in a closed container at 35°C.At equilibrium,what is the partial vapor pressure of toluene?
A)103 torr
B)91.634 torr
C)21.5 torr
D)68.9 torr
E)28.4 torr
A)103 torr
B)91.634 torr
C)21.5 torr
D)68.9 torr
E)28.4 torr

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
73
A solution of CF3H in H2CO is most likely to
A)be ideal.
B)not be ideal,but the deviations cannot be predicted.
C)show negative deviations from Raoult's law.
D)obey Raoult's law.
E)show positive deviations from Raoult's law.
A)be ideal.
B)not be ideal,but the deviations cannot be predicted.
C)show negative deviations from Raoult's law.
D)obey Raoult's law.
E)show positive deviations from Raoult's law.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following methods cannot be used to determine the molar mass of a nonelectrolyte?
A)measurement of the freezing-point depression of a solution of the compound
B)measurement of the boiling-point elevation of a solution of the compound
C)measurement of the pressure,temperature,volume,and mass of the compound in the gaseous state
D)measurement of the x-ray diffraction of a pure crystal of the compound
E)measurement of the osmotic pressure of a solution of the compound
A)measurement of the freezing-point depression of a solution of the compound
B)measurement of the boiling-point elevation of a solution of the compound
C)measurement of the pressure,temperature,volume,and mass of the compound in the gaseous state
D)measurement of the x-ray diffraction of a pure crystal of the compound
E)measurement of the osmotic pressure of a solution of the compound

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
75
A solution consisting of 0.276 mol of methylbenzene,C6H5CH3,in 242 g of nitrobenzene,C6H5NO2,freezes at -2.0 °C.Pure nitrobenzene freezes at 6.0°C.What is the freezing-point depression constant of nitrobenzene?
A)7.2°C/m
B)29°C/m
C)14.0°C/m
D)3.5°C/m
E)7.0°C/m
A)7.2°C/m
B)29°C/m
C)14.0°C/m
D)3.5°C/m
E)7.0°C/m

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
76
Benzene,C6H6,and toluene,C6H5CH3,form ideal solutions.At 35°C the vapor pressure of benzene is 160.torr and that of toluene is 50.0 torr.In an experiment,3.9 mol of benzene and 5.7 mol of toluene are placed in a closed container at 35°C and allowed to come to equilibrium.What is the mole fraction of toluene in the vapor phase?
A)0.52
B)0.69
C)0.31
D)0.12
E)0.60
A)0.52
B)0.69
C)0.31
D)0.12
E)0.60

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
77
When 1 mol of a nonvolatile nonelectrolyte is dissolved in 3 mol of a solvent,the vapor pressure of the solution compared with that of the pure solvent is
A)1/4.
B)4/5.
C)1/2.
D)1/5.
E)3/4.
A)1/4.
B)4/5.
C)1/2.
D)1/5.
E)3/4.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
78
A solute added to a solvent raises the boiling point of the solution because
A)the temperature to cause boiling must be great enough to boil not only the solvent but also the solute.
B)the solute particles raise the solvent's vapor pressure,thus requiring a higher temperature to cause boiling.
C)the solute increases the volume of the solution,and an increase in volume requires an increase in the temperature to reach the boiling point (derived from PV = nRT).
D)the solute particles lower the solvent's vapor pressure,thus requiring a higher temperature to cause boiling.
E)two of these explanations are correct.
A)the temperature to cause boiling must be great enough to boil not only the solvent but also the solute.
B)the solute particles raise the solvent's vapor pressure,thus requiring a higher temperature to cause boiling.
C)the solute increases the volume of the solution,and an increase in volume requires an increase in the temperature to reach the boiling point (derived from PV = nRT).
D)the solute particles lower the solvent's vapor pressure,thus requiring a higher temperature to cause boiling.
E)two of these explanations are correct.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
79
The fact that the boiling point of a pure solvent is lower than the boiling point of a solution of the same solvent is a direct consequence of the
A)freezing-point depression of the solution.
B)vapor pressure of the solution being higher than the vapor pressure of the pure solvent.
C)osmotic pressure of the solvent being lower than the osmotic pressure of the solution.
D)vapor pressure of the solution being lower than the vapor pressure of the pure solvent.
E)osmotic pressure of the solvent being higher than the osmotic pressure of the solution.
A)freezing-point depression of the solution.
B)vapor pressure of the solution being higher than the vapor pressure of the pure solvent.
C)osmotic pressure of the solvent being lower than the osmotic pressure of the solution.
D)vapor pressure of the solution being lower than the vapor pressure of the pure solvent.
E)osmotic pressure of the solvent being higher than the osmotic pressure of the solution.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
80
A solution of two liquids,A and B,shows negative deviation from Raoult's law.This means that
A)the two liquids have a positive heat of solution.
B)molecules of A interact more strongly with B than A molecules interact with A or B molecules interact with B.
C)molecules of A interact strongly with other A-type molecules.
D)molecules of A interact weakly,if at all,with B molecules.
E)molecules of A hinder the strong interaction between B molecules.
A)the two liquids have a positive heat of solution.
B)molecules of A interact more strongly with B than A molecules interact with A or B molecules interact with B.
C)molecules of A interact strongly with other A-type molecules.
D)molecules of A interact weakly,if at all,with B molecules.
E)molecules of A hinder the strong interaction between B molecules.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck


