Exam 12: Solutions
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
Consider the following gas-aqueous liquid equilibrium for a closed system at a constant temperature.
O2(g) 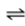 O2(aq)
What is the effect on the equilibrium composition of the liquid when the partial pressure of O2 gas above the liquid is decreased?
O2(aq)
What is the effect on the equilibrium composition of the liquid when the partial pressure of O2 gas above the liquid is decreased?
Free
(Multiple Choice)
5.0/5  (37)
(37)
Correct Answer:
A
To determine the molar mass of a small protein in the range of 20,000-40,000 g/mol,it would be best to measure
Free
(Multiple Choice)
4.9/5  (47)
(47)
Correct Answer:
D
If the solubility of O2 at 0.160 bar and 25°C is 6.65 g/100 g H2O,what is the solubility of O2 at a pressure of 1.84 bar and 25°C?
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
A
A solution consisting of 0.276 mol of methylbenzene,C6H5CH3,in 242 g of nitrobenzene,C6H5NO2,freezes at -2.0 °C.Pure nitrobenzene freezes at 6.0°C.What is the freezing-point depression constant of nitrobenzene?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following sets of conditions favors maximum solubility of an ionic solute in water?
(Multiple Choice)
4.8/5  (35)
(35)
Benzene,C6H6,and toluene,C6H5CH3,form ideal solutions.At 35°C the vapor pressure of benzene is 160.torr and that of toluene is 50.0 torr.In an experiment,3.9 mol of benzene and 5.7 mol of toluene are placed in a closed container at 35°C and allowed to come to equilibrium.What is the mole fraction of toluene in the vapor phase?
(Multiple Choice)
4.7/5  (28)
(28)
Which of the following sets of conditions favors maximum solubility of solute in solvent?
(Multiple Choice)
4.8/5  (38)
(38)
When 1 mol of a nonvolatile nonelectrolyte is dissolved in 3 mol of a solvent,the vapor pressure of the solution compared with that of the pure solvent is
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following statements best describes what happens when a small amount of solid rubidium chloride is dissolved in water?
(Multiple Choice)
4.9/5  (29)
(29)
What is the mole fraction of urea in a solution that contains 2.7 mol of urea and 4.6 mol of water?
(Multiple Choice)
4.9/5  (30)
(30)
A 3.140 molal solution of NaCl is prepared.How many grams of NaCl are present in a sample containing 2.692 kg of water?
(Multiple Choice)
4.9/5  (32)
(32)
What is the percent Na2CO3 by mass in a 1.92 molal aqueous solution?
(Multiple Choice)
4.7/5  (41)
(41)
Which of the following solutes in aqueous solution would be expected to exhibit the smallest freezing-point lowering (assuming ideal behavior)?
(Multiple Choice)
4.8/5  (31)
(31)
The molal boiling-point constant for water is 0.51°C/molal.The boiling point of a 1.00 m solution of Ca(NO3)2 should be increased by
(Multiple Choice)
4.7/5  (35)
(35)
A liquid-liquid solution is called an ideal solution if
I.it obeys PV = nRT.
II.it obeys Raoult's law.
III.solute-solute,solvent-solvent,and solute-solvent interactions are very similar.
IV.solute-solute,solvent-solvent,and solute-solvent interactions are quite different.
(Multiple Choice)
4.8/5  (31)
(31)
The fact that the boiling point of a pure solvent is lower than the boiling point of a solution of the same solvent is a direct consequence of the
(Multiple Choice)
4.9/5  (42)
(42)
What is the vapor pressure at 20°C of an ideal solution prepared by the addition of 1.36 g of the nonvolatile solute urea,CO(NH2)2,to 24.1 g of methanol,CH3OH? The vapor pressure of pure methanol at 20°C is 89.0 mmHg.
(Multiple Choice)
4.8/5  (30)
(30)
Calculate the molecular weight of a small protein if a 0.25-g sample dissolved in 180 mL of water has an osmotic pressure of 9.2 mmHg at 25°C.(R = 0.0821 L · atm/(K · mol))
(Multiple Choice)
4.9/5  (34)
(34)
A concentrated hydrofluoric acid solution is 49.0% HF by mass and has a density of 1.3406 g/mL at 25°C.What is the mass of HF per L of solution?
(Multiple Choice)
4.8/5  (31)
(31)
Showing 1 - 20 of 119
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)