Deck 13: Rates of Reaction
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/113
Play
Full screen (f)
Deck 13: Rates of Reaction
1
For the reaction of the ammonium ion with nitrous acid,the net reaction is
NH4+(aq)+ HNO2(aq)→ N2(g)+ 2H2O(l)+ H+(aq)
If the initial concentration of nitrous acid is 1.00 M and,after 17.8 s has elapsed,the concentration of nitrous acid has fallen to 0.72 M,what is the average rate of the reaction over this time interval?
A)-0.015 M/s
B)0.04 M/s
C)0.015 M/s
D)-0.04 M/s
E)0.096 M/s
NH4+(aq)+ HNO2(aq)→ N2(g)+ 2H2O(l)+ H+(aq)
If the initial concentration of nitrous acid is 1.00 M and,after 17.8 s has elapsed,the concentration of nitrous acid has fallen to 0.72 M,what is the average rate of the reaction over this time interval?
A)-0.015 M/s
B)0.04 M/s
C)0.015 M/s
D)-0.04 M/s
E)0.096 M/s
-0.015 M/s
2
Ozone reacts with nitrogen dioxide to produce oxygen and dinitrogen pentoxide according to the following chemical equation:
O3(g)+ 2NO2(g)→ O2(g)+ N2O5(g)
The rate law for this reaction is Rate = k[O3][NO2].If concentration is measured in moles per liter and time is measured in seconds,what are the units of k?
A)L ∙ mol-1 ∙ s
B)L2 ∙ mol-2 ∙ s-1
C)L ∙ mol-1 ∙ s-1
D)mol ∙ L-1 ∙ s-1
E)mol2 ∙ L-2 ∙ s-1
O3(g)+ 2NO2(g)→ O2(g)+ N2O5(g)
The rate law for this reaction is Rate = k[O3][NO2].If concentration is measured in moles per liter and time is measured in seconds,what are the units of k?
A)L ∙ mol-1 ∙ s
B)L2 ∙ mol-2 ∙ s-1
C)L ∙ mol-1 ∙ s-1
D)mol ∙ L-1 ∙ s-1
E)mol2 ∙ L-2 ∙ s-1
L ∙ mol-1 ∙ s-1
3
In the reaction 2H2O2(aq)→ 2H2O(l)+ O2(g),the initial concentration of H2O2 is 0.565 M and,17.0 seconds later,the concentration of H2O2 is 0.361 M.What is the average rate of reaction over this time interval?
A)0.012779 M/s
B)0.012608 M/s
C)0.0212 M/s
D)0.00619 M/s
E)0.00615 M/s
A)0.012779 M/s
B)0.012608 M/s
C)0.0212 M/s
D)0.00619 M/s
E)0.00615 M/s
0.00619 M/s
4
Which of the following statements is incorrect?
A)The rates of most chemical reactions change with time.
B)The rate constant for a reaction can be changed by changing the temperature.
C)The rate constant is dependent on the reactant concentrations.
D)In a series of stepwise reactions,the rate-determining step is the slowest one.
E)The rate of a catalyzed reaction is dependent on the concentration of the catalyst.
A)The rates of most chemical reactions change with time.
B)The rate constant for a reaction can be changed by changing the temperature.
C)The rate constant is dependent on the reactant concentrations.
D)In a series of stepwise reactions,the rate-determining step is the slowest one.
E)The rate of a catalyzed reaction is dependent on the concentration of the catalyst.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
5
A rate constant for a particular reaction is 0.0070 M-1·s−1.What is the overall order of this reaction?
A)2
B)3
C)4
D)0
E)1
A)2
B)3
C)4
D)0
E)1

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
6
If the rate law for a reaction is
Rate = k[ClO3-][I-][H+]2
What are the units of k when the unit of time is seconds and the unit of concentration is moles per liter?
A)(L ∙ s)/mol
B)mol2/(L2 ∙ s)
C)mol/(L ∙ s)
D)L2/(mol2 ∙ s)
E)L3/(mol3 ∙ s)
Rate = k[ClO3-][I-][H+]2
What are the units of k when the unit of time is seconds and the unit of concentration is moles per liter?
A)(L ∙ s)/mol
B)mol2/(L2 ∙ s)
C)mol/(L ∙ s)
D)L2/(mol2 ∙ s)
E)L3/(mol3 ∙ s)

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
7
For the hypothetical reaction A + 2B → 2C + D,the initial rate of disappearance of A is 2.0 × 10-2 mol/(L ∙ s).What is the initial rate of disappearance of B?
A)8.0 × 10-2 mol/(L ∙ s)
B)4 × 10-2 mol/(L ∙ s)
C)1.4 × 10-1 mol/(L ∙ s)
D)4.0 × 10-4 mol/(L ∙ s)
E)1.4 × 10-2 mol/(L ∙ s)
A)8.0 × 10-2 mol/(L ∙ s)
B)4 × 10-2 mol/(L ∙ s)
C)1.4 × 10-1 mol/(L ∙ s)
D)4.0 × 10-4 mol/(L ∙ s)
E)1.4 × 10-2 mol/(L ∙ s)

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements is always true?
A)Exothermic reactions have lower activation energies than endothermic reactions.
B)The rate of a catalyzed reaction is independent of the concentration of the catalyst.
C)The rate for a reaction depends on the concentrations of all the reactants.
D)The rate constant is independent of the concentrations of the reacting species.
E)The rate law can be determined from the stoichiometric equation.
A)Exothermic reactions have lower activation energies than endothermic reactions.
B)The rate of a catalyzed reaction is independent of the concentration of the catalyst.
C)The rate for a reaction depends on the concentrations of all the reactants.
D)The rate constant is independent of the concentrations of the reacting species.
E)The rate law can be determined from the stoichiometric equation.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements concerning the experimental determination of reaction rates is incorrect?
A)To determine reaction rates one of the reactants must be colored.
B)Monitoring changes in reactant or product physical properties is a convenient way to determine reaction rates.
C)Analysis of samples withdrawn from the reaction solution at varying times is useful for slow reactions.
D)Instrumental methods,such as visible spectroscopy,may be used to continuously measure changes in reactants or products.
E)Any method of analysis that can determine product or reactant concentrations during the course of the reaction can potentially be used to determine reaction rates.
A)To determine reaction rates one of the reactants must be colored.
B)Monitoring changes in reactant or product physical properties is a convenient way to determine reaction rates.
C)Analysis of samples withdrawn from the reaction solution at varying times is useful for slow reactions.
D)Instrumental methods,such as visible spectroscopy,may be used to continuously measure changes in reactants or products.
E)Any method of analysis that can determine product or reactant concentrations during the course of the reaction can potentially be used to determine reaction rates.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
10
For the reaction
6CH2O(aq)+ 4NH3(aq)→ (CH2)6N4(aq)+ 6H2O(l)
The rate of the reaction may be expressed as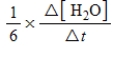
)What is an equivalent expression for the rate of the reaction?
A)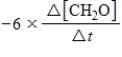
B)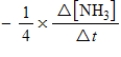
C)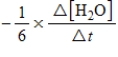
D)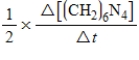
E)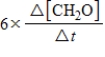
6CH2O(aq)+ 4NH3(aq)→ (CH2)6N4(aq)+ 6H2O(l)
The rate of the reaction may be expressed as
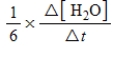
)What is an equivalent expression for the rate of the reaction?
A)
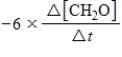
B)
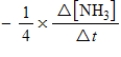
C)
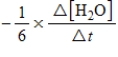
D)
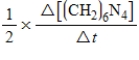
E)
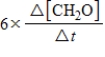

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
11
For which of the following hypothetical rate laws would the units of the rate constant have the general form M-3·time−1?
A)rate = k[A]4
B)rate = k
C)rate = k[A]
D)rate = k[A]2
E)rate = k[A]3
A)rate = k[A]4
B)rate = k
C)rate = k[A]
D)rate = k[A]2
E)rate = k[A]3

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
12
Consider the reaction
AA + bB![<strong>Consider the reaction AA + bB DD + eE C = catalyst The rate law is Rate = k[A]<sup>q</sup>[B]<sup>r</sup>[C]s Which of the following statements is incorrect?</strong> A)The exponents q and r are always equal to the coefficients a and b,respectively. B)The overall reaction order is q + r + s. C)The exponent s must be determined experimentally. D)The symbol k represents the rate constant. E)The exponents q,r,and s are often integers.](https://storage.examlex.com/TB2288/11ea7a3a_9f02_bd68_a82d_0d266c0241dc_TB2288_11.jpg)
DD + eE C = catalyst
The rate law is
Rate = k[A]q[B]r[C]s
Which of the following statements is incorrect?
A)The exponents q and r are always equal to the coefficients a and b,respectively.
B)The overall reaction order is q + r + s.
C)The exponent s must be determined experimentally.
D)The symbol k represents the rate constant.
E)The exponents q,r,and s are often integers.
AA + bB
![<strong>Consider the reaction AA + bB DD + eE C = catalyst The rate law is Rate = k[A]<sup>q</sup>[B]<sup>r</sup>[C]s Which of the following statements is incorrect?</strong> A)The exponents q and r are always equal to the coefficients a and b,respectively. B)The overall reaction order is q + r + s. C)The exponent s must be determined experimentally. D)The symbol k represents the rate constant. E)The exponents q,r,and s are often integers.](https://storage.examlex.com/TB2288/11ea7a3a_9f02_bd68_a82d_0d266c0241dc_TB2288_11.jpg)
DD + eE C = catalyst
The rate law is
Rate = k[A]q[B]r[C]s
Which of the following statements is incorrect?
A)The exponents q and r are always equal to the coefficients a and b,respectively.
B)The overall reaction order is q + r + s.
C)The exponent s must be determined experimentally.
D)The symbol k represents the rate constant.
E)The exponents q,r,and s are often integers.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
13
The hypochlorite ion oxidizes the iodide ion in aqueous solution as represented by the following equation:
OCl-(aq)+ I-(aq)→ OI-(aq)+ Cl-(aq)
The rate law for this reaction is Rate = k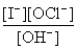
)If time is measured in seconds and concentration is measured in moles per liter,what are the units for k?
A)mol2/(L2 ∙ s)
B)L/(mol ∙ s)
C)1/s
D)L2/(mol2 ∙ s)
E)mol/(L ∙ s)
OCl-(aq)+ I-(aq)→ OI-(aq)+ Cl-(aq)
The rate law for this reaction is Rate = k
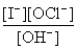
)If time is measured in seconds and concentration is measured in moles per liter,what are the units for k?
A)mol2/(L2 ∙ s)
B)L/(mol ∙ s)
C)1/s
D)L2/(mol2 ∙ s)
E)mol/(L ∙ s)

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
14
For the reaction
IO3-(aq)+ 5I-(aq)+ 6H+(aq)→ 3I2(aq)+ 3H2O(l)
The rate of disappearance of I O3-(aq)at a particular time and concentration is 2.5× 10-3 mol/(L · s).What is the rate of appearance of I2(aq)?
A)8.3 × 10-3 mol/(L ∙ s)
B)3 × 10-3 mol/(L ∙ s)
C)-8 × 10-3 mol/(L ∙ s)
D)8 × 10-3 mol/(L ∙ s)
E)1 × 10-3 mol/(L ∙ s)
IO3-(aq)+ 5I-(aq)+ 6H+(aq)→ 3I2(aq)+ 3H2O(l)
The rate of disappearance of I O3-(aq)at a particular time and concentration is 2.5× 10-3 mol/(L · s).What is the rate of appearance of I2(aq)?
A)8.3 × 10-3 mol/(L ∙ s)
B)3 × 10-3 mol/(L ∙ s)
C)-8 × 10-3 mol/(L ∙ s)
D)8 × 10-3 mol/(L ∙ s)
E)1 × 10-3 mol/(L ∙ s)

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
15
For a certain first-order reaction with the general form aA → products,the rate is 0.32 M·s−1 when the concentration of the reactant is 0.27 M.What is the rate constant for this reaction?
A)0.22 s−1
B)1.1 s−1
C)0.32 s−1
D)3.10 s−1
E)4.3 s−1
A)0.22 s−1
B)1.1 s−1
C)0.32 s−1
D)3.10 s−1
E)4.3 s−1

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
16
The oxidation of ammonia produces nitrogen and water via the following reaction:
4NH3(g)+ 3O2(g)→ 2N2(g)+ 6H2O(l)
Suppose the rate of formation of H2O(l)is 3.0 mol/(L ∙ s).Which of the following statements is true?
A)The rate of consumption of NH3 is 2.0 mol/(L ∙ s).
B)The rate of consumption of O2 is 2.0 mol/(L ∙ s).
C)The rate of formation of N2 is 1.3 mol/(L ∙ s).
D)The rate of formation of N2 is 2.0 mol/(L ∙ s).
E)The rate of consumption of NH3 is 0.50 mol/(L ∙ s).
4NH3(g)+ 3O2(g)→ 2N2(g)+ 6H2O(l)
Suppose the rate of formation of H2O(l)is 3.0 mol/(L ∙ s).Which of the following statements is true?
A)The rate of consumption of NH3 is 2.0 mol/(L ∙ s).
B)The rate of consumption of O2 is 2.0 mol/(L ∙ s).
C)The rate of formation of N2 is 1.3 mol/(L ∙ s).
D)The rate of formation of N2 is 2.0 mol/(L ∙ s).
E)The rate of consumption of NH3 is 0.50 mol/(L ∙ s).

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements is true concerning the reaction given below?
2H2S(g)+ O2(g)→ 2S(s)+ 2H2O(g)
A)The rate law is Rate = k[H2S]2[O2].
B)The reaction is second-order in H2S(g)and first-order in O2(g).
C)The reaction is first-order in H2S(g)and second-order in O2(g).
D)The rate law is Rate = k[H2S][O2].
E)The rate law may be determined only by experiment.
2H2S(g)+ O2(g)→ 2S(s)+ 2H2O(g)
A)The rate law is Rate = k[H2S]2[O2].
B)The reaction is second-order in H2S(g)and first-order in O2(g).
C)The reaction is first-order in H2S(g)and second-order in O2(g).
D)The rate law is Rate = k[H2S][O2].
E)The rate law may be determined only by experiment.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
18
For the reaction
2N2O5(g)→ 4NO2(g)+ O2(g)
Which of the following expressions is equal to the rate of the reaction?
A)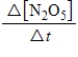
B)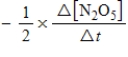
C)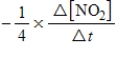
D)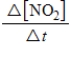
E)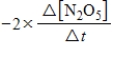
2N2O5(g)→ 4NO2(g)+ O2(g)
Which of the following expressions is equal to the rate of the reaction?
A)
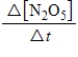
B)
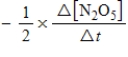
C)
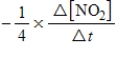
D)
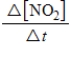
E)
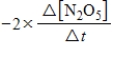

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following experimental methods cannot be used to measure the rate of a reaction?
A)measurement of the absorbance of a colored reactant with time
B)measurement of the change in the partial pressure of a gas-phase product over time
C)measurement of the equilibrium concentration of an acidic product via titration with a strong base
D)measurement of the absorbance of a colored product with time
E)measurement of the change in the partial pressure of a gas-phase reactant over time
A)measurement of the absorbance of a colored reactant with time
B)measurement of the change in the partial pressure of a gas-phase product over time
C)measurement of the equilibrium concentration of an acidic product via titration with a strong base
D)measurement of the absorbance of a colored product with time
E)measurement of the change in the partial pressure of a gas-phase reactant over time

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
20
For a second-order reaction,what are the possible units of the rate constant?
A)L-1 ∙ s-1
B)mol ∙ L-1 ∙ s-1
C)s
D)L.mol-1·s-1
E)mol ∙ L-1
A)L-1 ∙ s-1
B)mol ∙ L-1 ∙ s-1
C)s
D)L.mol-1·s-1
E)mol ∙ L-1

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
21
In aqueous solution,iodine reacts with acetone as represented by the following equation:
I2(aq)+ CH3COCH3(aq)→ CH3COCH2I(aq)+ H+(aq)+ I-(aq)
The experimental rate law is Rate = k[H+][CH3COCH3].According to the information above,an increase in the hydrogen ion concentration has what effect on the reaction?
A)It decreases the rate of the reaction.
B)It increases the rate of the reaction.
C)It decreases the value of the equilibrium constant.
D)It increases the value of the equilibrium constant.
E)It does not affect the rate of the reaction.
I2(aq)+ CH3COCH3(aq)→ CH3COCH2I(aq)+ H+(aq)+ I-(aq)
The experimental rate law is Rate = k[H+][CH3COCH3].According to the information above,an increase in the hydrogen ion concentration has what effect on the reaction?
A)It decreases the rate of the reaction.
B)It increases the rate of the reaction.
C)It decreases the value of the equilibrium constant.
D)It increases the value of the equilibrium constant.
E)It does not affect the rate of the reaction.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
22
The following data were obtained in a kinetics study of the hypothetical reaction A + B + C → products.
[A]0 (M)
[B]0 (M)
[C]0 (M)
Initial Rate (10-3 M/s)
0)4
0)4
0)2
160
0)2
0)4
0)4
80
0)6
0)1
0)2
15
0)2
0)1
0)2
5
0)2
0)2
0)4
20
Using the initial-rate method,what is the order of the reaction with respect to C?
A)zero-order
B)first-order
C)third-order
D)second-order
E)impossible to tell from the data given
[A]0 (M)
[B]0 (M)
[C]0 (M)
Initial Rate (10-3 M/s)
0)4
0)4
0)2
160
0)2
0)4
0)4
80
0)6
0)1
0)2
15
0)2
0)1
0)2
5
0)2
0)2
0)4
20
Using the initial-rate method,what is the order of the reaction with respect to C?
A)zero-order
B)first-order
C)third-order
D)second-order
E)impossible to tell from the data given

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
23
The reaction
2H2(g)+ 2NO(g)→ 2H2O(g)+ N2(g)
Is first-order in H2 and second-order in NO at a particular temperature.What is the rate law?
A)Rate = k[H2]2[NO]2
B)Rate = k[H2][NO]2
C)Rate = k[H2][NO]
D)Rate = k[H2O]2[N2]
E)Rate = k[H2]2[NO]
2H2(g)+ 2NO(g)→ 2H2O(g)+ N2(g)
Is first-order in H2 and second-order in NO at a particular temperature.What is the rate law?
A)Rate = k[H2]2[NO]2
B)Rate = k[H2][NO]2
C)Rate = k[H2][NO]
D)Rate = k[H2O]2[N2]
E)Rate = k[H2]2[NO]

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
24
If a reaction is first-order with respect to a particular reactant,when the concentration of that reactant is increased by a factor of 2,the reaction rate will _____.
A)increase by a factor of 2.
B)remain constant.
C)decrease by a factor of .
.
D)increase by a factor of 8.
E)increase by a factor of 4.
A)increase by a factor of 2.
B)remain constant.
C)decrease by a factor of
 .
.D)increase by a factor of 8.
E)increase by a factor of 4.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
25
The reaction between selenous acid and the iodide ion in acid solution is
H2SeO3(aq)+ 6I-(aq)+ 4H+(aq)→ Se(s)+ 2I3-(aq)+ 3H2O(l)
The data in the following table were measured at 0°C.
Experiment
[H2SeO3]0 (M)
[H+]0 (M)
[I-]0 (M)
Initial Rate [mol/(L ∙ s)]
1
1)00 × 10-4
2)00 × 10-2
3)00 × 10-2
5)30 × 10-7
2
2)00 × 10-4
2)00 × 10-2
3)00 × 10-2
1)06 × 10-6
3
3)00 × 10-4
4)00 × 10-2
3)00 × 10-2
6)36 × 10-6
4
3)00 × 10-4
8)00 × 10-2
3)00 × 10-2
2)54 × 10-5
5
3)00 × 10-4
8)00 × 10-2
6)00 × 10-2
2)04 × 10-4
6
2)00 × 10-4
2)00 × 10-2
6)00 × 10-2
8)48 × 10-6
Tripling the initial concentration of H2SeO3 while holding the initial concentrations of H+ and I- constant increases the rate of the reaction by a factor of
A)8.
B)4.
C)3.
D)2.
E)1.
H2SeO3(aq)+ 6I-(aq)+ 4H+(aq)→ Se(s)+ 2I3-(aq)+ 3H2O(l)
The data in the following table were measured at 0°C.
Experiment
[H2SeO3]0 (M)
[H+]0 (M)
[I-]0 (M)
Initial Rate [mol/(L ∙ s)]
1
1)00 × 10-4
2)00 × 10-2
3)00 × 10-2
5)30 × 10-7
2
2)00 × 10-4
2)00 × 10-2
3)00 × 10-2
1)06 × 10-6
3
3)00 × 10-4
4)00 × 10-2
3)00 × 10-2
6)36 × 10-6
4
3)00 × 10-4
8)00 × 10-2
3)00 × 10-2
2)54 × 10-5
5
3)00 × 10-4
8)00 × 10-2
6)00 × 10-2
2)04 × 10-4
6
2)00 × 10-4
2)00 × 10-2
6)00 × 10-2
8)48 × 10-6
Tripling the initial concentration of H2SeO3 while holding the initial concentrations of H+ and I- constant increases the rate of the reaction by a factor of
A)8.
B)4.
C)3.
D)2.
E)1.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
26
Nitrosyl chloride is produced from the reaction of nitrogen monoxide and chlorine:
2NO(g)+ Cl2(g)→ 2NOCl(g)
The following initial rates at a given temperature were obtained for the concentrations listed below.
Experiment
Initial Rate (mol·L-1·h-1)
[NO]0 (mol·L-1)
[Cl2]0 (mol·L-1)
1
2)21
0)25
0)25
2
19)89
0)75
0)25
3
6)63
0)25
0)75
From the data,what is the experimental rate law?
A)Rate = k[Cl2]
B)Rate = k[NO]
C)Rate = k[NO][Cl2]2
D)Rate = k[NO]2[Cl2]
E)Rate = k[NO][Cl2]1/2
2NO(g)+ Cl2(g)→ 2NOCl(g)
The following initial rates at a given temperature were obtained for the concentrations listed below.
Experiment
Initial Rate (mol·L-1·h-1)
[NO]0 (mol·L-1)
[Cl2]0 (mol·L-1)
1
2)21
0)25
0)25
2
19)89
0)75
0)25
3
6)63
0)25
0)75
From the data,what is the experimental rate law?
A)Rate = k[Cl2]
B)Rate = k[NO]
C)Rate = k[NO][Cl2]2
D)Rate = k[NO]2[Cl2]
E)Rate = k[NO][Cl2]1/2

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
27
The rate law for the chemical reaction
5Br-(aq)+ BrO3-(aq)+ 6H+(aq)→ 3Br2(aq)+ 3H2O(l)
Has been determined experimentally to be Rate = k[Br-][BrO3-][H+]2.What is the overall order of the reaction?
A)3
B)5
C)4
D)2
E)1
5Br-(aq)+ BrO3-(aq)+ 6H+(aq)→ 3Br2(aq)+ 3H2O(l)
Has been determined experimentally to be Rate = k[Br-][BrO3-][H+]2.What is the overall order of the reaction?
A)3
B)5
C)4
D)2
E)1

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
28
For the reaction A + B + C → products,the following initial-rate data were obtained.
[A]0 (mol/L)
[B]0 (mol/L)
[C]0 (mol/L)
Initial Rate (mol/(L ∙ s))
0)40
0)40
0)20
0)0160
0)20
0)40
0)40
0)0080
0)60
0)10
0)20
0)0015
0)20
0)10
0)20
0)0005
0)20
0)20
0)40
0)0020
What are the reaction orders with respect to A,B,and C,respectively?
A)0,1,1
B)1,2,1
C)1,1,1
D)1,2,0
E)0,2,1
[A]0 (mol/L)
[B]0 (mol/L)
[C]0 (mol/L)
Initial Rate (mol/(L ∙ s))
0)40
0)40
0)20
0)0160
0)20
0)40
0)40
0)0080
0)60
0)10
0)20
0)0015
0)20
0)10
0)20
0)0005
0)20
0)20
0)40
0)0020
What are the reaction orders with respect to A,B,and C,respectively?
A)0,1,1
B)1,2,1
C)1,1,1
D)1,2,0
E)0,2,1

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
29
For the reaction
(CH3)3CCl(aq)+ OH-(aq)→ (CH3)3COH(aq)+ Cl-(aq)
It is found experimentally that doubling the initial concentration of (CH3)3CCl causes the initial reaction rate to double,but doubling the initial concentration of OH- has no effect on the rate.What is the rate law?
A)Rate = k[(CH3)3CCl]2[OH-]
B)Rate = k[(CH3)3CCl][OH-]
C)Rate = k![<strong>For the reaction (CH<sub>3</sub>)<sub>3</sub>CCl(aq)+ OH<sup>-</sup>(aq)→ (CH<sub>3</sub>)<sub>3</sub>COH(aq)+ Cl<sup>-</sup>(aq) It is found experimentally that doubling the initial concentration of (CH<sub>3</sub>)<sub>3</sub>CCl causes the initial reaction rate to double,but doubling the initial concentration of OH<sup>-</sup> has no effect on the rate.What is the rate law?</strong> A)Rate = k[(CH<sub>3</sub>)<sub>3</sub>CCl]<sup>2</sup>[OH<sup>-</sup>] B)Rate = k[(CH<sub>3</sub>)<sub>3</sub>CCl][OH<sup>-</sup>] C)Rate = k D)Rate = k[(CH<sub>3</sub>)<sub>3</sub>COH][Cl<sup>-</sup>] E)Rate = k[(CH<sub>3</sub>)<sub>3</sub>CCl]](https://storage.examlex.com/TB2288/11ea7a3a_9f04_e04c_a82d_1385bfd9350f_TB2288_11.jpg)
D)Rate = k[(CH3)3COH][Cl-]
E)Rate = k[(CH3)3CCl]
(CH3)3CCl(aq)+ OH-(aq)→ (CH3)3COH(aq)+ Cl-(aq)
It is found experimentally that doubling the initial concentration of (CH3)3CCl causes the initial reaction rate to double,but doubling the initial concentration of OH- has no effect on the rate.What is the rate law?
A)Rate = k[(CH3)3CCl]2[OH-]
B)Rate = k[(CH3)3CCl][OH-]
C)Rate = k
![<strong>For the reaction (CH<sub>3</sub>)<sub>3</sub>CCl(aq)+ OH<sup>-</sup>(aq)→ (CH<sub>3</sub>)<sub>3</sub>COH(aq)+ Cl<sup>-</sup>(aq) It is found experimentally that doubling the initial concentration of (CH<sub>3</sub>)<sub>3</sub>CCl causes the initial reaction rate to double,but doubling the initial concentration of OH<sup>-</sup> has no effect on the rate.What is the rate law?</strong> A)Rate = k[(CH<sub>3</sub>)<sub>3</sub>CCl]<sup>2</sup>[OH<sup>-</sup>] B)Rate = k[(CH<sub>3</sub>)<sub>3</sub>CCl][OH<sup>-</sup>] C)Rate = k D)Rate = k[(CH<sub>3</sub>)<sub>3</sub>COH][Cl<sup>-</sup>] E)Rate = k[(CH<sub>3</sub>)<sub>3</sub>CCl]](https://storage.examlex.com/TB2288/11ea7a3a_9f04_e04c_a82d_1385bfd9350f_TB2288_11.jpg)
D)Rate = k[(CH3)3COH][Cl-]
E)Rate = k[(CH3)3CCl]

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
30
The reaction between selenous acid and the iodide ion in acid solution is
H2SeO3(aq)+ 6I-(aq)+ 4H+(aq)→ Se(s)+ 2I3-(aq)+ 3H2O(l)
The data in the following table were measured at 0°C.
Experiment
[H2SeO3]0 (M)
[H+]0 (M)
[I-]0 (M)
Initial Rate [mol/(L ∙ s)]
1
1)00 × 10-4
2)00 × 10-2
3)00 × 10-2
5)30 × 10-7
2
2)00 × 10-4
2)00 × 10-2
3)00 × 10-2
1)06 × 10-6
3
3)00 × 10-4
4)00 × 10-2
3)00 × 10-2
6)36 × 10-6
4
3)00 × 10-4
8)00 × 10-2
3)00 × 10-2
2)54 × 10-5
5
3)00 × 10-4
8)00 × 10-2
6)00 × 10-2
2)04 × 10-4
6
2)00 × 10-4
2)00 × 10-2
6)00 × 10-2
8)48 × 10-6
What is the rate constant for this reaction?
A)1.5 × 104 L5/(mol5 ∙ s)
B)1.5 × 1010 L5/(mol5 ∙ s)
C)4.9 × 105 L5/(mol5 ∙ s)
D)294 L5/(mol5 ∙ s)
E)8.8 L5/(mol5 ∙ s)
H2SeO3(aq)+ 6I-(aq)+ 4H+(aq)→ Se(s)+ 2I3-(aq)+ 3H2O(l)
The data in the following table were measured at 0°C.
Experiment
[H2SeO3]0 (M)
[H+]0 (M)
[I-]0 (M)
Initial Rate [mol/(L ∙ s)]
1
1)00 × 10-4
2)00 × 10-2
3)00 × 10-2
5)30 × 10-7
2
2)00 × 10-4
2)00 × 10-2
3)00 × 10-2
1)06 × 10-6
3
3)00 × 10-4
4)00 × 10-2
3)00 × 10-2
6)36 × 10-6
4
3)00 × 10-4
8)00 × 10-2
3)00 × 10-2
2)54 × 10-5
5
3)00 × 10-4
8)00 × 10-2
6)00 × 10-2
2)04 × 10-4
6
2)00 × 10-4
2)00 × 10-2
6)00 × 10-2
8)48 × 10-6
What is the rate constant for this reaction?
A)1.5 × 104 L5/(mol5 ∙ s)
B)1.5 × 1010 L5/(mol5 ∙ s)
C)4.9 × 105 L5/(mol5 ∙ s)
D)294 L5/(mol5 ∙ s)
E)8.8 L5/(mol5 ∙ s)

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
31
Two substances A and B react with each other in such a way that one-half of A remains after 25 min and one-fourth of A remains after 50 min.Doubling the concentration of B while keeping the concentration of A fixed doubles the rate of the reaction.This reaction is
A)first-order in both A and B.
B)zero-order in both A and B.
C)second-order in A and first-order in B.
D)first-order in A and second-order in B.
E)second-order in both A and B.
A)first-order in both A and B.
B)zero-order in both A and B.
C)second-order in A and first-order in B.
D)first-order in A and second-order in B.
E)second-order in both A and B.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
32
The following data were obtained for the hypothetical reaction 2A + B → products.

What is the overall order of this reaction?
A)4
B)1/2
C)0
D)3
E)1

What is the overall order of this reaction?
A)4
B)1/2
C)0
D)3
E)1

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
33
If a reaction is zero-order in a reactant,when the concentration of the reactant is decreased by a factor of 2,the reaction rate will
A)quadruple.
B)decrease by a factor of 1/2.
C)remain constant.
D)decrease by a factor of 1/4.
E)double.
A)quadruple.
B)decrease by a factor of 1/2.
C)remain constant.
D)decrease by a factor of 1/4.
E)double.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
34
The rate law for the hydrolysis of thioacetamide (CH3CSNH2),
CH3CSNH2(aq)+ H2O(l)→ H2S(g)+ CH3CONH2(aq)
Is Rate = k[H+][CH3CSNH2].If,during the course of the reaction,some solid sodium hydroxide is added to the reaction mixture,then
A)the reaction rate decreases,but k remains the same.
B)the reaction rate remains the same,but k decreases.
C)the reaction rate increases,but k remains the same.
D)there is no change in the reaction rate or the rate constant.
E)the reaction rate remains the same,but k increases.
CH3CSNH2(aq)+ H2O(l)→ H2S(g)+ CH3CONH2(aq)
Is Rate = k[H+][CH3CSNH2].If,during the course of the reaction,some solid sodium hydroxide is added to the reaction mixture,then
A)the reaction rate decreases,but k remains the same.
B)the reaction rate remains the same,but k decreases.
C)the reaction rate increases,but k remains the same.
D)there is no change in the reaction rate or the rate constant.
E)the reaction rate remains the same,but k increases.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
35
The acid-catalyzed reaction of acetone,CH3COCH3,with iodine can be represented by the following net reaction:
CH3COCH3 + I2![<strong>The acid-catalyzed reaction of acetone,CH<sub>3</sub>COCH<sub>3</sub>,with iodine can be represented by the following net reaction: CH<sub>3</sub>COCH<sub>3</sub> + I<sub>2</sub> CH<sub>2</sub>ICOCH<sub>3</sub> + H<sup>+</sup> + I<sup>-</sup> It is found experimentally that the rate law for this reaction is Rate = k[CH<sub>3</sub>COCH<sub>3</sub>][H<sup>+</sup>].Suppose that in trial 1,the initial rate of the reaction is measured with the initial concentrations of acetone,iodine,and hydrogen ion all equal to 0.10 M.Then,in trial 2,the initial rate of the reaction is measured with the initial concentrations all equal to 0.20 M.The initial rate of trial 2 will be larger than the initial rate of trial 1 by a factor of</strong> A)4. B)16. C)8. D)2. E)64.](https://storage.examlex.com/TB2288/11ea7a3a_9f05_a39d_a82d_ff58c247adb6_TB2288_11.jpg)
CH2ICOCH3 + H+ + I-
It is found experimentally that the rate law for this reaction is Rate = k[CH3COCH3][H+].Suppose that in trial 1,the initial rate of the reaction is measured with the initial concentrations of acetone,iodine,and hydrogen ion all equal to 0.10 M.Then,in trial 2,the initial rate of the reaction is measured with the initial concentrations all equal to 0.20 M.The initial rate of trial 2 will be larger than the initial rate of trial 1 by a factor of
A)4.
B)16.
C)8.
D)2.
E)64.
CH3COCH3 + I2
![<strong>The acid-catalyzed reaction of acetone,CH<sub>3</sub>COCH<sub>3</sub>,with iodine can be represented by the following net reaction: CH<sub>3</sub>COCH<sub>3</sub> + I<sub>2</sub> CH<sub>2</sub>ICOCH<sub>3</sub> + H<sup>+</sup> + I<sup>-</sup> It is found experimentally that the rate law for this reaction is Rate = k[CH<sub>3</sub>COCH<sub>3</sub>][H<sup>+</sup>].Suppose that in trial 1,the initial rate of the reaction is measured with the initial concentrations of acetone,iodine,and hydrogen ion all equal to 0.10 M.Then,in trial 2,the initial rate of the reaction is measured with the initial concentrations all equal to 0.20 M.The initial rate of trial 2 will be larger than the initial rate of trial 1 by a factor of</strong> A)4. B)16. C)8. D)2. E)64.](https://storage.examlex.com/TB2288/11ea7a3a_9f05_a39d_a82d_ff58c247adb6_TB2288_11.jpg)
CH2ICOCH3 + H+ + I-
It is found experimentally that the rate law for this reaction is Rate = k[CH3COCH3][H+].Suppose that in trial 1,the initial rate of the reaction is measured with the initial concentrations of acetone,iodine,and hydrogen ion all equal to 0.10 M.Then,in trial 2,the initial rate of the reaction is measured with the initial concentrations all equal to 0.20 M.The initial rate of trial 2 will be larger than the initial rate of trial 1 by a factor of
A)4.
B)16.
C)8.
D)2.
E)64.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
36
For the reaction between nitrogen monoxide and chlorine to produce nitrosyl chloride,2NO(g)+ Cl2(g)→ 2NOCl(g),it is found that tripling the initial concentration of both reactants increases the initial rate by a factor of 27.If only the initial concentration of chlorine is tripled,the initial rate increases by a factor of 3.What is the order of the reaction with respect to Cl2?
A)1/2
B)1
C)2
D)3
E)0
A)1/2
B)1
C)2
D)3
E)0

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
37
The balanced chemical equation and rate law for the reaction between NO(g)and H2(g)at a particular temperature are
2NO(g)+ 2H2(g)→ N2(g)+ 2H2O(g)
Rate = k[NO]2[H2]
What is the reaction order with respect to nitric oxide?
A)4
B)0
C)1
D)3
E)2
2NO(g)+ 2H2(g)→ N2(g)+ 2H2O(g)
Rate = k[NO]2[H2]
What is the reaction order with respect to nitric oxide?
A)4
B)0
C)1
D)3
E)2

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
38
The hypochlorite ion oxidizes the iodide ion in aqueous solution as represented by the following equation:
OCl-(aq)+ I-(aq)→ OI-(aq)+ Cl-(aq)
The rate law for this reaction is Rate = k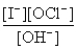
)The overall reaction order and the order with respect to OH- are
A)2 and -1.
B)0 and -1.
C)0 and 1.
D)2 and 1.
E)1 and -1.
OCl-(aq)+ I-(aq)→ OI-(aq)+ Cl-(aq)
The rate law for this reaction is Rate = k
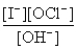
)The overall reaction order and the order with respect to OH- are
A)2 and -1.
B)0 and -1.
C)0 and 1.
D)2 and 1.
E)1 and -1.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
39
The reaction between selenous acid and the iodide ion in acid solution is
H2SeO3(aq)+ 6I-(aq)+ 4H+(aq)→ Se(s)+ 2I3-(aq)+ 3H2O(l)
The data in the following table were measured at 0°C.
Experiment
[H2SeO3]0 (M)
[H+]0 (M)
[I-]0 (M)
Initial Rate [mol/(L ∙ s)]
1
1)00 × 10-4
2)00 × 10-2
3)00 × 10-2
5)30 × 10-7
2
2)00 × 10-4
2)00 × 10-2
3)00 × 10-2
1)06 × 10-6
3
3)00 × 10-4
4)00 × 10-2
3)00 × 10-2
6)36 × 10-6
4
3)00 × 10-4
8)00 × 10-2
3)00 × 10-2
2)54 × 10-5
5
3)00 × 10-4
8)00 × 10-2
6)00 × 10-2
2)04 × 10-4
6
2)00 × 10-4
2)00 × 10-2
6)00 × 10-2
8)48 × 10-6
The overall order of this reaction is
A)4.
B)6.
C)2.
D)8.
E)3.
H2SeO3(aq)+ 6I-(aq)+ 4H+(aq)→ Se(s)+ 2I3-(aq)+ 3H2O(l)
The data in the following table were measured at 0°C.
Experiment
[H2SeO3]0 (M)
[H+]0 (M)
[I-]0 (M)
Initial Rate [mol/(L ∙ s)]
1
1)00 × 10-4
2)00 × 10-2
3)00 × 10-2
5)30 × 10-7
2
2)00 × 10-4
2)00 × 10-2
3)00 × 10-2
1)06 × 10-6
3
3)00 × 10-4
4)00 × 10-2
3)00 × 10-2
6)36 × 10-6
4
3)00 × 10-4
8)00 × 10-2
3)00 × 10-2
2)54 × 10-5
5
3)00 × 10-4
8)00 × 10-2
6)00 × 10-2
2)04 × 10-4
6
2)00 × 10-4
2)00 × 10-2
6)00 × 10-2
8)48 × 10-6
The overall order of this reaction is
A)4.
B)6.
C)2.
D)8.
E)3.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
40
The rate law for the reaction between chlorine and nitric oxide,
2NO(g)+ Cl2(g)→ 2NOCl(g)
Is Rate = k[NO]2[Cl2].Which of the following changes will not alter the initial rate of the reaction?
A)increasing the concentration of NOCl
B)decreasing the volume of the reaction system
C)running the reaction in a solvent rather than in the gas phase
D)increasing the volume of the reaction system
E)increasing the concentration of chlorine gas
2NO(g)+ Cl2(g)→ 2NOCl(g)
Is Rate = k[NO]2[Cl2].Which of the following changes will not alter the initial rate of the reaction?
A)increasing the concentration of NOCl
B)decreasing the volume of the reaction system
C)running the reaction in a solvent rather than in the gas phase
D)increasing the volume of the reaction system
E)increasing the concentration of chlorine gas

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
41
The gas-phase decomposition of N2O5 is a first-order process with a rate constant of 1.50 × 10-3 s-1 at 55°C.The decomposition reaction is
N2O5(g)→ 2NO2(g)+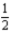
O2(g)
If 8.0 g of N2O5 is placed in vessel 1 and 16.0 g of N2O5 in vessel 2 and the vessels are at the same temperature (55°C)and the same pressure,how much time is required for half of the N2O5 to decompose in each vessel?
A)Vessel 1 requires the same amount of time as vessel 2.
B)Vessel 1 requires twice as much time as vessel 2.
C)Vessel 1 requires three times as much time as vessel 2.
D)Vessel 1 requires four times as much time as vessel 2.
E)Vessel 2 requires twice as much time as vessel 1.
N2O5(g)→ 2NO2(g)+
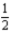
O2(g)
If 8.0 g of N2O5 is placed in vessel 1 and 16.0 g of N2O5 in vessel 2 and the vessels are at the same temperature (55°C)and the same pressure,how much time is required for half of the N2O5 to decompose in each vessel?
A)Vessel 1 requires the same amount of time as vessel 2.
B)Vessel 1 requires twice as much time as vessel 2.
C)Vessel 1 requires three times as much time as vessel 2.
D)Vessel 1 requires four times as much time as vessel 2.
E)Vessel 2 requires twice as much time as vessel 1.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
42
The reaction between selenous acid and the iodide ion in acid solution is
H2SeO3(aq)+ 6I-(aq)+ 4H+(aq)→ Se(s)+ 2I3-(aq)+ 3H2O(l)
The data in the following table were measured at 0°C.
Experiment
[H2SeO3]0 (M)
[H+]0 (M)
[I-]0 (M)
Initial Rate [mol/(L ∙ s)]
1
1)00 × 10-4
2)00 × 10-2
3)00 × 10-2
5)30 × 10-7
2
2)00 × 10-4
2)00 × 10-2
3)00 × 10-2
1)06 × 10-6
3
3)00 × 10-4
4)00 × 10-2
3)00 × 10-2
6)36 × 10-6
4
3)00 × 10-4
8)00 × 10-2
3)00 × 10-2
2)54 × 10-5
5
3)00 × 10-4
8)00 × 10-2
6)00 × 10-2
2)04 × 10-4
6
2)00 × 10-4
2)00 × 10-2
6)00 × 10-2
8)48 × 10-6
Tripling the initial concentration of I- while holding the initial concentrations of H2SeO3 and H+ constant increases the initial rate of the reaction by a factor of
A)27.
B)9.
C)3.
D)8.
E)6.
H2SeO3(aq)+ 6I-(aq)+ 4H+(aq)→ Se(s)+ 2I3-(aq)+ 3H2O(l)
The data in the following table were measured at 0°C.
Experiment
[H2SeO3]0 (M)
[H+]0 (M)
[I-]0 (M)
Initial Rate [mol/(L ∙ s)]
1
1)00 × 10-4
2)00 × 10-2
3)00 × 10-2
5)30 × 10-7
2
2)00 × 10-4
2)00 × 10-2
3)00 × 10-2
1)06 × 10-6
3
3)00 × 10-4
4)00 × 10-2
3)00 × 10-2
6)36 × 10-6
4
3)00 × 10-4
8)00 × 10-2
3)00 × 10-2
2)54 × 10-5
5
3)00 × 10-4
8)00 × 10-2
6)00 × 10-2
2)04 × 10-4
6
2)00 × 10-4
2)00 × 10-2
6)00 × 10-2
8)48 × 10-6
Tripling the initial concentration of I- while holding the initial concentrations of H2SeO3 and H+ constant increases the initial rate of the reaction by a factor of
A)27.
B)9.
C)3.
D)8.
E)6.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
43
A reaction that is second-order in one reactant has a rate constant of 3.8 × 10-2 L/(mol ∙ s).If the initial concentration of the reactant is 0.280 mol/L,how long will it take for the concentration to become 0.140 mol/L?
A)180 s
B)47 s
C)930 s
D)18 s
E)93 s
A)180 s
B)47 s
C)930 s
D)18 s
E)93 s

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
44
For the hypothetical first-order reaction A → products,k = 0.0822 s-1.If the initial concentration of A is 0.372 M,how long would it take for A to be 28.2% consumed?
A)8.43 s
B)4.03 s
C)12 s
D)32.7 s
E)15.3 s
A)8.43 s
B)4.03 s
C)12 s
D)32.7 s
E)15.3 s

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
45
For the hypothetical second-order reaction A → products,k = 0.379 M-1 s-1.If the initial concentration of A is 0.799 M,how long would it take for A to be 39.7% consumed?
A)5.01 s
B)3.3129 s
C)1.33 s
D)2 s
E)2.43 s
A)5.01 s
B)3.3129 s
C)1.33 s
D)2 s
E)2.43 s

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
46
A chemical reaction that is first-order in X is observed to have a rate constant of 2.00 × 10-2 s-1.If the initial concentration of X is 1.0 M,what is the concentration of X after 196 s?
A)0.20 M
B)0.020 M
C)50 M
D)0.61 M
E)0.98 M
A)0.20 M
B)0.020 M
C)50 M
D)0.61 M
E)0.98 M

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
47
The radioactive nuclide 63Ni decays by a first-order process via the emission of a beta particle.The 63Ni nuclide has a half-life of 100.years.How long will it take for 71% of 63Ni to decay?
A)49.4 years
B)21.4 years
C)0.857 years
D)178 years
E)77.5 years
A)49.4 years
B)21.4 years
C)0.857 years
D)178 years
E)77.5 years

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
48
The nuclide 188W decays by a first-order process with a rate constant of 1.0 × 10-2 d-1.How long will it take for 88% of the initial amount of 188W to be consumed?
A)6 d
B)240 d
C)12 d
D)210 d
E)92 d
A)6 d
B)240 d
C)12 d
D)210 d
E)92 d

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
49
The reaction between selenous acid and the iodide ion in acid solution is
H2SeO3(aq)+ 6I-(aq)+ 4H+(aq)→ Se(s)+ 2I3-(aq)+ 3H2O(l)
The data in the following table were measured at 0°C.
Experiment
[H2SeO3]0 (M)
[H+]0 (M)
[I-]0 (M)
Initial Rate [mol/(L ∙ s)]
1
1)00 × 10-4
2)00 × 10-2
3)00 × 10-2
5)30 × 10-7
2
2)00 × 10-4
2)00 × 10-2
3)00 × 10-2
1)06 × 10-6
3
3)00 × 10-4
4)00 × 10-2
3)00 × 10-2
6)36 × 10-6
4
3)00 × 10-4
8)00 × 10-2
3)00 × 10-2
2)54 × 10-5
5
3)00 × 10-4
8)00 × 10-2
6)00 × 10-2
2)04 × 10-4
6
2)00 × 10-4
2)00 × 10-2
6)00 × 10-2
8)48 × 10-6
Tripling the initial concentration of H+ while holding the initial concentrations of H2SeO3 and I- constant increases the initial rate of the reaction by a factor of
A)9.
B)16.
C)32.
D)2.
E)4.
H2SeO3(aq)+ 6I-(aq)+ 4H+(aq)→ Se(s)+ 2I3-(aq)+ 3H2O(l)
The data in the following table were measured at 0°C.
Experiment
[H2SeO3]0 (M)
[H+]0 (M)
[I-]0 (M)
Initial Rate [mol/(L ∙ s)]
1
1)00 × 10-4
2)00 × 10-2
3)00 × 10-2
5)30 × 10-7
2
2)00 × 10-4
2)00 × 10-2
3)00 × 10-2
1)06 × 10-6
3
3)00 × 10-4
4)00 × 10-2
3)00 × 10-2
6)36 × 10-6
4
3)00 × 10-4
8)00 × 10-2
3)00 × 10-2
2)54 × 10-5
5
3)00 × 10-4
8)00 × 10-2
6)00 × 10-2
2)04 × 10-4
6
2)00 × 10-4
2)00 × 10-2
6)00 × 10-2
8)48 × 10-6
Tripling the initial concentration of H+ while holding the initial concentrations of H2SeO3 and I- constant increases the initial rate of the reaction by a factor of
A)9.
B)16.
C)32.
D)2.
E)4.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
50
The half-life of a reaction is
A)twice as long for a second-order reaction as it is for a first-order reaction.
B)one-half of the time the reaction will take to go to completion.
C)how long the reaction can run before stopping.
D)the time it takes for the amount of product formed to equal half the initial amount of reactant.
E)the time it takes for the reactant concentration to decrease to one-half of its initial value.
A)twice as long for a second-order reaction as it is for a first-order reaction.
B)one-half of the time the reaction will take to go to completion.
C)how long the reaction can run before stopping.
D)the time it takes for the amount of product formed to equal half the initial amount of reactant.
E)the time it takes for the reactant concentration to decrease to one-half of its initial value.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
51
At a given temperature,a first-order reaction has a rate constant of 2.5 × 10-3 s-1.How long will it take for the reaction to be 35% complete?
A)410 s
B)1600 s
C)1400 s
D)74 s
E)170 s
A)410 s
B)1600 s
C)1400 s
D)74 s
E)170 s

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following is not a correct representation of the integrated rate expression for a first-order reaction?
A)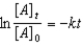
B)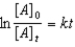
C)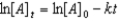
D)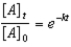
E)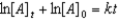
A)
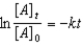
B)
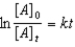
C)
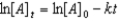
D)
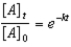
E)
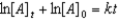

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
53
At 500oC,cyclopropane (C3H6)reacts to form its isomer,propene (C3H6).The reaction is first-order,and the rate constant is 6.7 × 10-4 s-1.If the initial concentration of cyclopropane is 0.500 M and the initial concentration of propene is 0,determine the time required for the concentration of propene to reach 0.100 M.
A)3.4 × 103 s
B)3.3 × 102 s
C)1.2 × 104 s
D)7.5 × 102 s
E)2.4 × 103 s
A)3.4 × 103 s
B)3.3 × 102 s
C)1.2 × 104 s
D)7.5 × 102 s
E)2.4 × 103 s

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
54
The reaction 3NO → N2O + NO2 is found to obey the rate law Rate = k[NO]2.If the first half-life of the reaction is found to be 2.0 s,what is the length of the fourth half-life?
A)4.0 s
B)8.0 s
C)2.0 s
D)16.0 s
E)12.0 s
A)4.0 s
B)8.0 s
C)2.0 s
D)16.0 s
E)12.0 s

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
55
A second-order reaction starts with an initial concentration of 0.100 mol/L of the reactant.If the rate constant is 2.4× 10-2 L/(mol ∙ s),what is the time required to decrease the initial concentration to 0.050 mol/L?
A)410 s
B)620 s
C)28.9 s
D)2.08 s
E)1200 s
A)410 s
B)620 s
C)28.9 s
D)2.08 s
E)1200 s

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
56
The reaction A → products is first-order in A.If the concentration of A is cut in half,the half-life of the reaction will
A)decrease by a factor of 1/2.
B)double.
C)decrease by a factor of 1/4.
D)remain constant.
E)quadruple.
A)decrease by a factor of 1/2.
B)double.
C)decrease by a factor of 1/4.
D)remain constant.
E)quadruple.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
57
For which order reaction is the half-life of the reaction proportional to 1/k (k is the rate constant)?
A)second-order reaction only
B)first-order reaction only
C)zero-order reaction only
D)all of the above
E)none of the above
A)second-order reaction only
B)first-order reaction only
C)zero-order reaction only
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
58
The nuclide 96Nb decays by a first-order process with a rate constant of 2.96 × 10-2 h-1.How long will it take for 86.0% of the initial amount of 96Nb to be consumed?
A)33.7 h
B)66.4 h
C)30 h
D)5.09 h
E)4.72 h
A)33.7 h
B)66.4 h
C)30 h
D)5.09 h
E)4.72 h

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
59
Dinitrogen tetroxide decomposes to form nitrogen dioxide in a second-order reaction:
N2O4(g)→ 2NO2(g)
At 400.0 K,the rate constant for this reaction has been measured to be 2.9 × 108 L/(mol ∙ s).Suppose 0.742 mol of N2O4(g)is placed in a sealed 34.3-L container at 400.0 K and allowed to react.What is the total pressure inside the vessel after 49.6 ns has elapsed? (R= 0.0821 (L ∙ atm)/(K ∙ mol))
A)0.879 atm
B)0.7138 atm
C)0.541 atm
D)2.13 atm
E)1.42 atm
N2O4(g)→ 2NO2(g)
At 400.0 K,the rate constant for this reaction has been measured to be 2.9 × 108 L/(mol ∙ s).Suppose 0.742 mol of N2O4(g)is placed in a sealed 34.3-L container at 400.0 K and allowed to react.What is the total pressure inside the vessel after 49.6 ns has elapsed? (R= 0.0821 (L ∙ atm)/(K ∙ mol))
A)0.879 atm
B)0.7138 atm
C)0.541 atm
D)2.13 atm
E)1.42 atm

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following corresponds to the correct integrated expression for a second-order reaction?
A)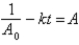
B)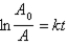
C)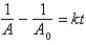
D)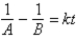
E)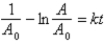
A)
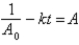
B)
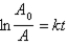
C)
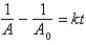
D)
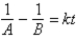
E)
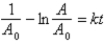

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
61
For the hypothetical reaction A → products,the concentration of A was monitored over time.From the following graph,what is the rate constant for the decomposition of A? 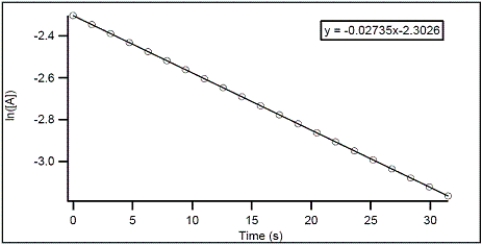
A)-0.02735 s-1
B)2.3026 s-1
C)-2.3026 s-1
D)0.02735 s-1
E)0.01188 s-1

A)-0.02735 s-1
B)2.3026 s-1
C)-2.3026 s-1
D)0.02735 s-1
E)0.01188 s-1

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following corresponds to the correct equation for the half-life of a first-order reaction?
A)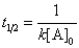
B)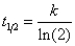
C)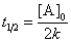
D)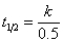
E)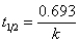
A)
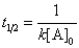
B)
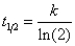
C)
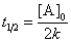
D)
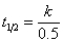
E)
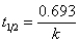

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following is not a postulate of collision theory?
A)Reactant molecules must collide to react.
B)Reactant molecules must collide with a certain minimum energy in order to form products.
C)Reactant molecules must collide with the correct orientation in order to form products.
D)The rate constant is directly proportional to the energy of activation.
E)The maximum in the potential energy curve,the activation energy,is determined by the structure of the activated complex or transition state.
A)Reactant molecules must collide to react.
B)Reactant molecules must collide with a certain minimum energy in order to form products.
C)Reactant molecules must collide with the correct orientation in order to form products.
D)The rate constant is directly proportional to the energy of activation.
E)The maximum in the potential energy curve,the activation energy,is determined by the structure of the activated complex or transition state.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
64
For the following reaction producing 1 mol of oxygen gas at a particular temperature,ΔH = -200 kJ.
NO(g)+ O3(g)→ NO2(g)+ O2(g)
The activation energy is 11 kJ/mol.What is the activation energy for the reverse reaction?
A)11 kJ/mol
B)200 kJ/mol
C)222 kJ/mol
D)188 kJ/mol
E)211 kJ/mol
NO(g)+ O3(g)→ NO2(g)+ O2(g)
The activation energy is 11 kJ/mol.What is the activation energy for the reverse reaction?
A)11 kJ/mol
B)200 kJ/mol
C)222 kJ/mol
D)188 kJ/mol
E)211 kJ/mol

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
65
The main reason for the increase in reaction rate with temperature is that
A)the fraction of high-energy molecules increases exponentially with temperature.
B)the activation energy increases rapidly with temperature.
C)a 10°C temperature rise results in the rate doubling.
D)there is a dramatic increase in the number of collisions.
E)heat acts as a catalyst.
A)the fraction of high-energy molecules increases exponentially with temperature.
B)the activation energy increases rapidly with temperature.
C)a 10°C temperature rise results in the rate doubling.
D)there is a dramatic increase in the number of collisions.
E)heat acts as a catalyst.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
66
For the first-order reaction
1/2 N2O4(g)→ NO2(g); ΔH = 28.6 kJ
The activation energy is 53.7 kJ/mol.What is the activation energy for the reverse reaction?
A)15.2 kJ/mol
B)82.3 kJ/mol
C)-53.7 kJ/mol
D)25.1 kJ/mol
E)53.7 kJ/mol
1/2 N2O4(g)→ NO2(g); ΔH = 28.6 kJ
The activation energy is 53.7 kJ/mol.What is the activation energy for the reverse reaction?
A)15.2 kJ/mol
B)82.3 kJ/mol
C)-53.7 kJ/mol
D)25.1 kJ/mol
E)53.7 kJ/mol

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following statements best describes the condition(s)needed for a successful formation for a product according to the collision model?
A)The relative orientation of the particles has an effect only if the kinetic energy of the particles is below some minimum value.
B)The collision must involve a sufficient amount of energy,provided from the motion of the particles,to overcome the activation energy.
C)The relative orientation of the particles must allow for formation of the new bonds in the product.
D)The energy of the incoming particles must be above a certain minimum value,and the relative orientation of the particles must allow for formation of new bonds in the product.
E)The relative orientation of the particles has little or no effect on the formation of the product.
A)The relative orientation of the particles has an effect only if the kinetic energy of the particles is below some minimum value.
B)The collision must involve a sufficient amount of energy,provided from the motion of the particles,to overcome the activation energy.
C)The relative orientation of the particles must allow for formation of the new bonds in the product.
D)The energy of the incoming particles must be above a certain minimum value,and the relative orientation of the particles must allow for formation of new bonds in the product.
E)The relative orientation of the particles has little or no effect on the formation of the product.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
68
When the concentrations of the reactants are increased,the rate of the reaction increases.This is best explained by
A)an increase in the fraction of molecules that have enough energy to react.
B)an increase in the rate constant.
C)an increase in the average potential energy of the molecules.
D)an increase in the frequency of the molecular collisions.
E)an increase in the kinetic energy of the molecules.
A)an increase in the fraction of molecules that have enough energy to react.
B)an increase in the rate constant.
C)an increase in the average potential energy of the molecules.
D)an increase in the frequency of the molecular collisions.
E)an increase in the kinetic energy of the molecules.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
69
The rates of most chemical reactions are sensitive to a change in the temperature of the reaction system.The increase in rate as the temperature increases is best explained by
A)an increase in the collision frequency.
B)an increase in the number of high-energy molecules.
C)a decrease in the collision frequency.
D)an increase in the activation energy.
E)a decrease in the activation energy.
A)an increase in the collision frequency.
B)an increase in the number of high-energy molecules.
C)a decrease in the collision frequency.
D)an increase in the activation energy.
E)a decrease in the activation energy.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
70
For a certain reaction of the general form aA → products,the experimental data plotted as 1/[A] versus time is linear.The slope of this plot must equal
A)-1.
B)the rate constant.
C)one over the rate constant.
D)the negative of the rate constant.
E)1.
A)-1.
B)the rate constant.
C)one over the rate constant.
D)the negative of the rate constant.
E)1.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
71
The OH· radical disproportionates according to the elementary chemical reaction 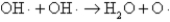 This reaction is second-order in OH·.The rate constant for the reaction is 2.0 × 10-12 cm3/molecules at room temperature.If the initial OH· concentration is 1.4 × 1013 molecules/cm3,what is the first half-life for the reaction?
This reaction is second-order in OH·.The rate constant for the reaction is 2.0 × 10-12 cm3/molecules at room temperature.If the initial OH· concentration is 1.4 × 1013 molecules/cm3,what is the first half-life for the reaction?
A)2.8 × 101 s
B)3.6 × 10-2 s
C)1.8 × 10-2 s
D)3.5 × 1011 s
E)7.1 × 10-14 s
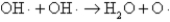 This reaction is second-order in OH·.The rate constant for the reaction is 2.0 × 10-12 cm3/molecules at room temperature.If the initial OH· concentration is 1.4 × 1013 molecules/cm3,what is the first half-life for the reaction?
This reaction is second-order in OH·.The rate constant for the reaction is 2.0 × 10-12 cm3/molecules at room temperature.If the initial OH· concentration is 1.4 × 1013 molecules/cm3,what is the first half-life for the reaction?A)2.8 × 101 s
B)3.6 × 10-2 s
C)1.8 × 10-2 s
D)3.5 × 1011 s
E)7.1 × 10-14 s

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
72
For a certain reaction of the general form aA → products,a plot of the experimental data as [A] versus time is linear.What is the reaction order with respect to reactant A?
A)zero
B)first
C)second
D)fourth
E)third
A)zero
B)first
C)second
D)fourth
E)third

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
73
For the hypothetical reaction aA → products,the concentration of A was monitored with time.Given the following graph of the experimental data,what is the rate constant for the loss of reactant A? 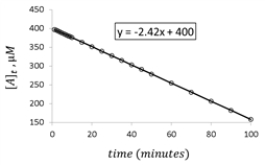
A)2.42 μΜ −1⋅min−1
B)400.00 μΜ −1⋅min−1
C)-2.42 μΜ −1⋅min−1
D)-400 μΜ −1⋅min−1
E)24.2 μΜ −1⋅min−1

A)2.42 μΜ −1⋅min−1
B)400.00 μΜ −1⋅min−1
C)-2.42 μΜ −1⋅min−1
D)-400 μΜ −1⋅min−1
E)24.2 μΜ −1⋅min−1

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
74
The rate constant for a first-order reaction is 1.5× 10-2 s-1 at 710 K and 4.1 × 10-2 s-1 at 884 K.What is the activation energy? (R = 8.31 J/(mol · K))
A)12 kJ/mol
B)13 kJ/mol
C)29 kJ/mol
D)3600 kJ/mol
E)30 kJ/mol
A)12 kJ/mol
B)13 kJ/mol
C)29 kJ/mol
D)3600 kJ/mol
E)30 kJ/mol

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
75
For the hypothetical reaction A → products,the concentration of A was monitored with time.From the following graph,what is the rate constant for the decomposition of A? 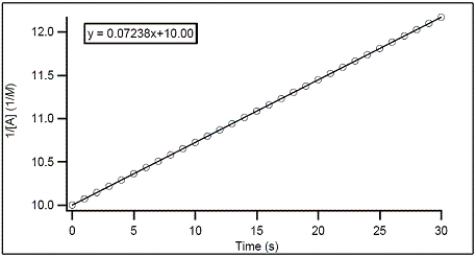
A)-0.07238 M-1 s-1
B)-10.00 M-1 s-1
C)0.07238 M-1 s-1
D)10.00 M-1 s-1
E)0.007238 M-1 s-1

A)-0.07238 M-1 s-1
B)-10.00 M-1 s-1
C)0.07238 M-1 s-1
D)10.00 M-1 s-1
E)0.007238 M-1 s-1

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
76
In a first-order reaction,the half-life is 139 minutes.What is the rate constant?
A)1.20 × 10-4 s-1
B)5770 s-1
C)0.299 s-1
D)4.99 × 10-3 s-1
E)8.31 × 10-5 s-1
A)1.20 × 10-4 s-1
B)5770 s-1
C)0.299 s-1
D)4.99 × 10-3 s-1
E)8.31 × 10-5 s-1

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
77
What would happen if the kinetic energy of the reactants were not enough to provide the needed activation energy?
A)The rate of the reaction would tend to increase.
B)The reactants would continue to exist in their present form.
C)The activated complex would be converted into products.
D)The products would be produced at a lower energy state.
E)The products would form at an unstable energy state.
A)The rate of the reaction would tend to increase.
B)The reactants would continue to exist in their present form.
C)The activated complex would be converted into products.
D)The products would be produced at a lower energy state.
E)The products would form at an unstable energy state.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
78
For the formation of 1 mol of nitrosyl chloride at a given temperature,ΔH = -38 kJ.
NO(g)+ 1/2 Cl2(g)→ NOCl(g)
The activation energy for this reaction is 69 kJ/mol.What is the activation energy for the reverse reaction?
A)69 kJ/mol
B)31 kJ/mol
C)107 kJ/mol
D)-38 kJ/mol
E)-107 kJ/mol
NO(g)+ 1/2 Cl2(g)→ NOCl(g)
The activation energy for this reaction is 69 kJ/mol.What is the activation energy for the reverse reaction?
A)69 kJ/mol
B)31 kJ/mol
C)107 kJ/mol
D)-38 kJ/mol
E)-107 kJ/mol

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
79
For the hypothetical reaction aA → products,the experimental data showed the following behavior (below).What is the reaction order with respect to reactant A? 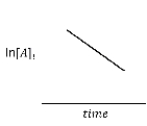
A)first-order
B)second-order
C)zero-order
D)third-order
E)fourth-order

A)first-order
B)second-order
C)zero-order
D)third-order
E)fourth-order

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
80
A first-order chemical reaction is observed to have a rate constant of 24 min-1.What is the corresponding half-life for the reaction?
A)1.7 s
B)1.7 min
C)35 min
D)2.5 s
E)34.3 s
A)1.7 s
B)1.7 min
C)35 min
D)2.5 s
E)34.3 s

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck


