Exam 13: Rates of Reaction
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
Dinitrogen tetroxide decomposes to form nitrogen dioxide in a second-order reaction:
N2O4(g)→ 2NO2(g)
At 400.0 K,the rate constant for this reaction has been measured to be 2.9 × 108 L/(mol ∙ s).Suppose 0.742 mol of N2O4(g)is placed in a sealed 34.3-L container at 400.0 K and allowed to react.What is the total pressure inside the vessel after 49.6 ns has elapsed? (R= 0.0821 (L ∙ atm)/(K ∙ mol))
Free
(Multiple Choice)
4.8/5  (42)
(42)
Correct Answer:
A
The following mechanism has been suggested for the reaction between nitrogen monoxide and oxygen:
NO(g)+ NO(g)→ N2O2(g)(fast)
N2O2(g)+ O2(g)→ 2NO2(g)(slow)
According to this mechanism,the experimental rate law is
Free
(Multiple Choice)
4.8/5  (28)
(28)
Correct Answer:
B
For the reaction of the ammonium ion with nitrous acid,the net reaction is
NH4+(aq)+ HNO2(aq)→ N2(g)+ 2H2O(l)+ H+(aq)
If the initial concentration of nitrous acid is 1.00 M and,after 17.8 s has elapsed,the concentration of nitrous acid has fallen to 0.72 M,what is the average rate of the reaction over this time interval?
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
A
In a chemical reaction at constant temperature,the addition of a catalyst
(Multiple Choice)
4.7/5  (27)
(27)
The oxidation of ammonia produces nitrogen and water via the following reaction:
4NH3(g)+ 3O2(g)→ 2N2(g)+ 6H2O(l)
Suppose the rate of formation of H2O(l)is 3.0 mol/(L ∙ s).Which of the following statements is true?
(Multiple Choice)
4.9/5  (31)
(31)
How many mechanistic steps are depicted by in this potential energy diagram for the decomposition of cyclobutane to ethylene? 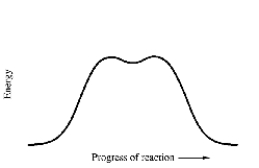
(Multiple Choice)
4.9/5  (36)
(36)
In aqueous solution,iodine reacts with acetone as represented by the following equation:
I2(aq)+ CH3COCH3(aq)→ CH3COCH2I(aq)+ H+(aq)+ I-(aq)
The experimental rate law is Rate = k[H+][CH3COCH3].According to the information above,an increase in the hydrogen ion concentration has what effect on the reaction?
(Multiple Choice)
4.8/5  (37)
(37)
The complete mechanism for a reaction is considered to occur in two steps,one of which is slow and the other fast:
A + B → C + D slow
A + C → E + F fast
What is the net chemical equation predicted by this mechanism?
(Multiple Choice)
4.9/5  (29)
(29)
For the reaction
6CH2O(aq)+ 4NH3(aq)→ (CH2)6N4(aq)+ 6H2O(l)
The rate of the reaction may be expressed as 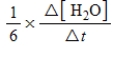 )What is an equivalent expression for the rate of the reaction?
)What is an equivalent expression for the rate of the reaction?
(Multiple Choice)
4.8/5  (25)
(25)
For a second-order reaction,what are the possible units of the rate constant?
(Multiple Choice)
4.7/5  (39)
(39)
Identify the rate equation of the termolecular elementary reaction given below.
A + B + C D + E
(Multiple Choice)
4.9/5  (36)
(36)
For the reaction between nitrogen monoxide and chlorine to produce nitrosyl chloride,2NO(g)+ Cl2(g)→ 2NOCl(g),it is found that tripling the initial concentration of both reactants increases the initial rate by a factor of 27.If only the initial concentration of chlorine is tripled,the initial rate increases by a factor of 3.What is the order of the reaction with respect to Cl2?
(Multiple Choice)
4.8/5  (26)
(26)
Consider the reaction
AA + bB ![Consider the reaction AA + bB DD + eE C = catalyst The rate law is Rate = k[A]<sup>q</sup>[B]<sup>r</sup>[C]s Which of the following statements is incorrect?](https://storage.examlex.com/TB2288/11ea7a3a_9f02_bd68_a82d_0d266c0241dc_TB2288_11.jpg) DD + eE C = catalyst
The rate law is
Rate = k[A]q[B]r[C]s
Which of the following statements is incorrect?
DD + eE C = catalyst
The rate law is
Rate = k[A]q[B]r[C]s
Which of the following statements is incorrect?
(Multiple Choice)
4.7/5  (33)
(33)
For the hypothetical second-order reaction A → products,k = 0.379 M-1 s-1.If the initial concentration of A is 0.799 M,how long would it take for A to be 39.7% consumed?
(Multiple Choice)
4.8/5  (31)
(31)
The following data were obtained for the hypothetical reaction 2A + B → products.
 What is the overall order of this reaction?
What is the overall order of this reaction?
(Multiple Choice)
4.9/5  (33)
(33)
The reaction between selenous acid and the iodide ion in acid solution is
H2SeO3(aq)+ 6I-(aq)+ 4H+(aq)→ Se(s)+ 2I3-(aq)+ 3H2O(l)
The data in the following table were measured at 0°C.
Experiment
[H2SeO3]0 (M)
[H+]0 (M)
[I-]0 (M)
Initial Rate [mol/(L ∙ s)]
1
1)00 × 10-4
2)00 × 10-2
3)00 × 10-2
5)30 × 10-7
2
2)00 × 10-4
2)00 × 10-2
3)00 × 10-2
1)06 × 10-6
3
3)00 × 10-4
4)00 × 10-2
3)00 × 10-2
6)36 × 10-6
4
3)00 × 10-4
8)00 × 10-2
3)00 × 10-2
2)54 × 10-5
5
3)00 × 10-4
8)00 × 10-2
6)00 × 10-2
2)04 × 10-4
6
2)00 × 10-4
2)00 × 10-2
6)00 × 10-2
8)48 × 10-6
Tripling the initial concentration of I- while holding the initial concentrations of H2SeO3 and H+ constant increases the initial rate of the reaction by a factor of
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following statements is true concerning the reaction given below?
2H2S(g)+ O2(g)→ 2S(s)+ 2H2O(g)
(Multiple Choice)
4.8/5  (35)
(35)
A proposed mechanism for the decomposition of N2O5 is as follows:
N2O5 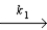 NO2 + NO3
Slow step
NO2 + NO3
NO2 + NO3
Slow step
NO2 + NO3 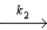 NO2 + O2 + NO
Fast step
NO + N2O5
NO2 + O2 + NO
Fast step
NO + N2O5 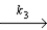 3NO2
Fast step
What is the rate law predicted by this mechanism?
3NO2
Fast step
What is the rate law predicted by this mechanism?
(Multiple Choice)
4.7/5  (43)
(43)
A suggested mechanism for the decomposition of ozone is as follows:
O3 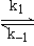 O2 + O fast equilibrium
O + O3
O2 + O fast equilibrium
O + O3 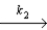 2O2 slow step
What is the rate law predicted by this mechanism?
2O2 slow step
What is the rate law predicted by this mechanism?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 1 - 20 of 113
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)