Deck 14: Chemical Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/97
Play
Full screen (f)
Deck 14: Chemical Equilibrium
1
Sulfur dioxide combines with O2 in the presence of a catalyst as represented by the equation
2SO2(g)+ O2(g)![<strong>Sulfur dioxide combines with O<sub>2</sub> in the presence of a catalyst as represented by the equation 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g) 2SO<sub>3</sub>(g) Suppose 0.10 mol of SO<sub>2</sub> and 0.10 mol of O<sub>2</sub> are added to a 1-L vessel.At equilibrium,which of the following conditions must be true?</strong> A)[O<sub>2</sub>] = 2[SO<sub>3</sub>] B)[SO<sub>2</sub>] = [O<sub>2</sub>] C)[SO<sub>2</sub>] > [O<sub>2</sub>] D)[SO<sub>2</sub>] < [O<sub>2</sub>] E)[SO<sub>2</sub>] = [O<sub>2</sub>] = [SO<sub>3</sub>]](https://storage.examlex.com/TB2288/11ea7a3a_9f12_4d1b_a82d_4379531168f5_TB2288_11.jpg)
2SO3(g)
Suppose 0.10 mol of SO2 and 0.10 mol of O2 are added to a 1-L vessel.At equilibrium,which of the following conditions must be true?
A)[O2] = 2[SO3]
B)[SO2] = [O2]
C)[SO2] > [O2]
D)[SO2] < [O2]
E)[SO2] = [O2] = [SO3]
2SO2(g)+ O2(g)
![<strong>Sulfur dioxide combines with O<sub>2</sub> in the presence of a catalyst as represented by the equation 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g) 2SO<sub>3</sub>(g) Suppose 0.10 mol of SO<sub>2</sub> and 0.10 mol of O<sub>2</sub> are added to a 1-L vessel.At equilibrium,which of the following conditions must be true?</strong> A)[O<sub>2</sub>] = 2[SO<sub>3</sub>] B)[SO<sub>2</sub>] = [O<sub>2</sub>] C)[SO<sub>2</sub>] > [O<sub>2</sub>] D)[SO<sub>2</sub>] < [O<sub>2</sub>] E)[SO<sub>2</sub>] = [O<sub>2</sub>] = [SO<sub>3</sub>]](https://storage.examlex.com/TB2288/11ea7a3a_9f12_4d1b_a82d_4379531168f5_TB2288_11.jpg)
2SO3(g)
Suppose 0.10 mol of SO2 and 0.10 mol of O2 are added to a 1-L vessel.At equilibrium,which of the following conditions must be true?
A)[O2] = 2[SO3]
B)[SO2] = [O2]
C)[SO2] > [O2]
D)[SO2] < [O2]
E)[SO2] = [O2] = [SO3]
[SO2] < [O2]
2
Nitrogen trifluoride decomposes to form nitrogen and fluorine gases according to the following equation:
2NF3(g)
N2(g)+ 3F2(g)
When 2.54 mol of NF3 is placed in a 6.00-L container and allowed to come to equilibrium at 800 K,the mixture is found to contain 0.0434 mol of N2.What is the value of Kp at this temperature? (R = 0.0821 L · atm · mol-1 · K-1)
A)1.54 × 10-5
B)1.91 × 10-3
C)1.78 × 10-3
D)1.59 × 10-5
E)4.43 × 10-7
2NF3(g)

N2(g)+ 3F2(g)
When 2.54 mol of NF3 is placed in a 6.00-L container and allowed to come to equilibrium at 800 K,the mixture is found to contain 0.0434 mol of N2.What is the value of Kp at this temperature? (R = 0.0821 L · atm · mol-1 · K-1)
A)1.54 × 10-5
B)1.91 × 10-3
C)1.78 × 10-3
D)1.59 × 10-5
E)4.43 × 10-7
1.91 × 10-3
3
Which of the following represents a dynamic equilibrium?
A)a stoppered flask half full of water
B)a coin spinning in mid-air
C)two people of equal mass balanced on the ends of a seesaw
D)an open pan of boiling water
E)an object traveling at a constant speed
A)a stoppered flask half full of water
B)a coin spinning in mid-air
C)two people of equal mass balanced on the ends of a seesaw
D)an open pan of boiling water
E)an object traveling at a constant speed
a stoppered flask half full of water
4
Which of the following statements is true in a reaction system at equilibrium?
A)The equilibrium constant is zero.
B)The number of collisions per unit time between reactants is equal to the number of collisions per unit time between products.
C)Reactants are reacting to form products at the same rate as products are reacting to form reactants.
D)Reactants and products are present in equimolar amounts.
E)The product of the concentrations of the products divided by the product of the concentrations of the reactants is always a constant.
A)The equilibrium constant is zero.
B)The number of collisions per unit time between reactants is equal to the number of collisions per unit time between products.
C)Reactants are reacting to form products at the same rate as products are reacting to form reactants.
D)Reactants and products are present in equimolar amounts.
E)The product of the concentrations of the products divided by the product of the concentrations of the reactants is always a constant.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
5
The reaction of a mixture of SO2 and O2 at a given temperature is represented by the equation
2SO2(g)+ O2(g)
2SO3(g)
When equilibrium is established,which of the following ratios is constant regardless of the initial concentrations of SO2 and O2?
A)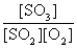
B)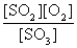
C)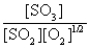
D)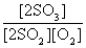
E)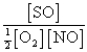
2SO2(g)+ O2(g)

2SO3(g)
When equilibrium is established,which of the following ratios is constant regardless of the initial concentrations of SO2 and O2?
A)
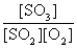
B)
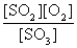
C)
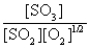
D)
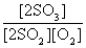
E)
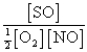

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
6
The Ostwald process converts ammonia (NH3)to nitric oxide (NO)by reaction with oxygen in the presence of a catalyst at high temperatures.In a test of the process a reaction vessel is initially charged with 4.80 mol NH3(g)and 5.80 mol O2(g),sealed,and heated at a fixed high temperature.When equilibrium is established the reaction mixture is analyzed and found to contain 3.80 mol NO(g).What is the quantity of NH3(g)in the equilibrium reaction mixture?
4NH3(g)+ 5O2(g)
4NO(g)+ 6H2O(g)
A)1 mol NH3(g)
B)8.6 mol NH3(g)
C)4.8 mol NH3(g)
D)1.05 mol NH3(g)
E)2 mol NH3(g)
4NH3(g)+ 5O2(g)

4NO(g)+ 6H2O(g)
A)1 mol NH3(g)
B)8.6 mol NH3(g)
C)4.8 mol NH3(g)
D)1.05 mol NH3(g)
E)2 mol NH3(g)

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
7
At 400 K,an equilibrium mixture of H2,I2,and HI consists of 0.025 mol H2,0.067 mol I2,and 0 mol HI in a 3.50-L flask.What is the value of Kp for the following equilibrium? (R = 0.0821 L ∙ atm/(K ∙ mol))
2HI(g) H2(g)+ I2(g)
H2(g)+ I2(g)
A)0
B)15
C)2
D)45
E)3
2HI(g)
 H2(g)+ I2(g)
H2(g)+ I2(g)A)0
B)15
C)2
D)45
E)3

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
8
For the reaction Br2(g)+ Cl2(g)  2BrCl(g),at equilibrium,it is found that the concentrations of Br2,Cl2,and BrCl are 0.484 M,0.105 M,and 1.24 × 10-3 M,respectively.What is the value of Kc?
2BrCl(g),at equilibrium,it is found that the concentrations of Br2,Cl2,and BrCl are 0.484 M,0.105 M,and 1.24 × 10-3 M,respectively.What is the value of Kc?
A)3.01 × 10-5
B)1.20 × 10-4
C)2.43 × 10-2
D)4.12 × 101
E)3.32 × 104
 2BrCl(g),at equilibrium,it is found that the concentrations of Br2,Cl2,and BrCl are 0.484 M,0.105 M,and 1.24 × 10-3 M,respectively.What is the value of Kc?
2BrCl(g),at equilibrium,it is found that the concentrations of Br2,Cl2,and BrCl are 0.484 M,0.105 M,and 1.24 × 10-3 M,respectively.What is the value of Kc?A)3.01 × 10-5
B)1.20 × 10-4
C)2.43 × 10-2
D)4.12 × 101
E)3.32 × 104

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
9
A 2.50-mol sample of HI is placed in a 1.00-L vessel at 460°C,and the reaction system is allowed to come to equilibrium.The HI partially decomposes,forming 0.190 mol H2 and 0.190 mol I2 at equilibrium.What is the equilibrium constant Kc for the following reaction at 460°C?
1/2 H2(g)+ 1/2 I2(g)
HI(g)
A)1.23 × 102
B)8.10 × 10-3
C)1.72 × 10-2
D)11.1
E)7.63
1/2 H2(g)+ 1/2 I2(g)

HI(g)
A)1.23 × 102
B)8.10 × 10-3
C)1.72 × 10-2
D)11.1
E)7.63

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
10
A sample of ammonia gas was allowed to come to equilibrium at 400 K.
2NH3(g)
N2(g)+ 3H2(g)
At equilibrium,it was found that the concentration of H2 was 0.0484 M,the concentration of N2 was 0.0161 M,and the concentration of NH3 was 0.295 M.What was the initial concentration of ammonia?
A)0.161 M
B)0.228 M
C)0.36 M
D)0.311 M
E)0.328 M
2NH3(g)

N2(g)+ 3H2(g)
At equilibrium,it was found that the concentration of H2 was 0.0484 M,the concentration of N2 was 0.0161 M,and the concentration of NH3 was 0.295 M.What was the initial concentration of ammonia?
A)0.161 M
B)0.228 M
C)0.36 M
D)0.311 M
E)0.328 M

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
11
The following reaction is investigated (assume an ideal gas mixture):
2N2O(g)+ N2H4(g)
3N2(g)+ 2H2O(g)
Initially there are 0.100 mol of N2O and 0.25 mol of N2H4,in a 10.0-L container.If there are 0.059 mol of N2O at equilibrium,how many moles of N2 are present at equilibrium?
A)4.1 × 10-2
B)1.2 × 10-1
C)6.2 × 10-2
D)2.1 × 10-2
E)none of these
2N2O(g)+ N2H4(g)

3N2(g)+ 2H2O(g)
Initially there are 0.100 mol of N2O and 0.25 mol of N2H4,in a 10.0-L container.If there are 0.059 mol of N2O at equilibrium,how many moles of N2 are present at equilibrium?
A)4.1 × 10-2
B)1.2 × 10-1
C)6.2 × 10-2
D)2.1 × 10-2
E)none of these

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following correctly describes the equilibrium constant for the gas-phase reaction between H2 and O2 to form gaseous H2O?
A)Kc =![<strong>Which of the following correctly describes the equilibrium constant for the gas-phase reaction between H<sub>2</sub> and O<sub>2</sub> to form gaseous H<sub>2</sub>O?</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = [H<sub>2</sub>O] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://storage.examlex.com/TB2288/11ea7a3a_9f14_2201_a82d_35de22d79a9d_TB2288_11.jpg)
B)Kc =![<strong>Which of the following correctly describes the equilibrium constant for the gas-phase reaction between H<sub>2</sub> and O<sub>2</sub> to form gaseous H<sub>2</sub>O?</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = [H<sub>2</sub>O] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://storage.examlex.com/TB2288/11ea7a3a_9f14_2202_a82d_e57010f529a6_TB2288_11.jpg)
C)Kc = [H2O]
D)Kc =![<strong>Which of the following correctly describes the equilibrium constant for the gas-phase reaction between H<sub>2</sub> and O<sub>2</sub> to form gaseous H<sub>2</sub>O?</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = [H<sub>2</sub>O] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://storage.examlex.com/TB2288/11ea7a3a_9f14_4913_a82d_9983b724de7b_TB2288_11.jpg)
E)Kc =![<strong>Which of the following correctly describes the equilibrium constant for the gas-phase reaction between H<sub>2</sub> and O<sub>2</sub> to form gaseous H<sub>2</sub>O?</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = [H<sub>2</sub>O] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://storage.examlex.com/TB2288/11ea7a3a_9f14_4914_a82d_4324e2c5e20c_TB2288_11.jpg)
A)Kc =
![<strong>Which of the following correctly describes the equilibrium constant for the gas-phase reaction between H<sub>2</sub> and O<sub>2</sub> to form gaseous H<sub>2</sub>O?</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = [H<sub>2</sub>O] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://storage.examlex.com/TB2288/11ea7a3a_9f14_2201_a82d_35de22d79a9d_TB2288_11.jpg)
B)Kc =
![<strong>Which of the following correctly describes the equilibrium constant for the gas-phase reaction between H<sub>2</sub> and O<sub>2</sub> to form gaseous H<sub>2</sub>O?</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = [H<sub>2</sub>O] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://storage.examlex.com/TB2288/11ea7a3a_9f14_2202_a82d_e57010f529a6_TB2288_11.jpg)
C)Kc = [H2O]
D)Kc =
![<strong>Which of the following correctly describes the equilibrium constant for the gas-phase reaction between H<sub>2</sub> and O<sub>2</sub> to form gaseous H<sub>2</sub>O?</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = [H<sub>2</sub>O] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://storage.examlex.com/TB2288/11ea7a3a_9f14_4913_a82d_9983b724de7b_TB2288_11.jpg)
E)Kc =
![<strong>Which of the following correctly describes the equilibrium constant for the gas-phase reaction between H<sub>2</sub> and O<sub>2</sub> to form gaseous H<sub>2</sub>O?</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = [H<sub>2</sub>O] D)K<sub>c</sub> = E)K<sub>c</sub> =](https://storage.examlex.com/TB2288/11ea7a3a_9f14_4914_a82d_4324e2c5e20c_TB2288_11.jpg)

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
13
A sample of ammonia gas was allowed to come to equilibrium at 400 K.
2NH3(g)
N2(g)+ 3H2(g)
At equilibrium,it was found that the concentration of H2 was 0.0551 M,the concentration of N2 was 0.0183 M,and the concentration of NH3 was 0.383 M.What is Kc for this equilibrium?
A)3.97 × 10-3
B)1.58 × 10-5
C)2.10 × 10-5
D)2.40 × 10-1
E)2.65 × 10-3
2NH3(g)

N2(g)+ 3H2(g)
At equilibrium,it was found that the concentration of H2 was 0.0551 M,the concentration of N2 was 0.0183 M,and the concentration of NH3 was 0.383 M.What is Kc for this equilibrium?
A)3.97 × 10-3
B)1.58 × 10-5
C)2.10 × 10-5
D)2.40 × 10-1
E)2.65 × 10-3

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
14
A 35.00-L vessel at 700 K initially contains HI(g)at a pressure of 5.80 atm; at equilibrium,it is found that the partial pressure of H2(g)is 0.56 atm.What is the partial pressure of HI(g)at equilibrium?
2HI(g)
H2(g)+ I2(g)
A)5.8 atm
B)5.23 atm
C)4.67 atm
D)6.36 atm
E)0.561 atm
2HI(g)

H2(g)+ I2(g)
A)5.8 atm
B)5.23 atm
C)4.67 atm
D)6.36 atm
E)0.561 atm

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
15
What is the balanced equation for the following equilibrium expression? 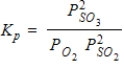
A)2SO2(g)+ O2(g) 2SO3(g)
2SO3(g)
B)2SO3(g) 2SO2(g)+ O2(g)
2SO2(g)+ O2(g)
C)2SO3(aq) 2SO2(aq)+ O2(aq)
2SO2(aq)+ O2(aq)
D)2SO2(aq)+ O2(aq) 2SO3(aq)
2SO3(aq)
E)SO2(g)+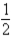 O2(g)
O2(g)  SO3(g)
SO3(g)
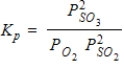
A)2SO2(g)+ O2(g)
 2SO3(g)
2SO3(g)B)2SO3(g)
 2SO2(g)+ O2(g)
2SO2(g)+ O2(g)C)2SO3(aq)
 2SO2(aq)+ O2(aq)
2SO2(aq)+ O2(aq)D)2SO2(aq)+ O2(aq)
 2SO3(aq)
2SO3(aq)E)SO2(g)+
 O2(g)
O2(g)  SO3(g)
SO3(g)
Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
16
What is the expression for Kc for the following equilibrium?
CaSO3(s)![<strong>What is the expression for K<sub>c</sub> for the following equilibrium? CaSO<sub>3</sub>(s) CaO(s)+ SO<sub>2</sub>(g)</strong> A)[CaO] [SO<sub>2</sub>] B)[SO<sub>2</sub>] C) D) E)](https://storage.examlex.com/TB2288/11ea7a3a_9f12_c252_a82d_f517eca7ddc6_TB2288_11.jpg)
CaO(s)+ SO2(g)
A)[CaO] [SO2]
B)[SO2]
C)![<strong>What is the expression for K<sub>c</sub> for the following equilibrium? CaSO<sub>3</sub>(s) CaO(s)+ SO<sub>2</sub>(g)</strong> A)[CaO] [SO<sub>2</sub>] B)[SO<sub>2</sub>] C) D) E)](https://storage.examlex.com/TB2288/11ea7a3a_9f12_e963_a82d_dd47e3637ced_TB2288_11.jpg)
D)![<strong>What is the expression for K<sub>c</sub> for the following equilibrium? CaSO<sub>3</sub>(s) CaO(s)+ SO<sub>2</sub>(g)</strong> A)[CaO] [SO<sub>2</sub>] B)[SO<sub>2</sub>] C) D) E)](https://storage.examlex.com/TB2288/11ea7a3a_9f12_e964_a82d_3d65c245f369_TB2288_11.jpg)
E)![<strong>What is the expression for K<sub>c</sub> for the following equilibrium? CaSO<sub>3</sub>(s) CaO(s)+ SO<sub>2</sub>(g)</strong> A)[CaO] [SO<sub>2</sub>] B)[SO<sub>2</sub>] C) D) E)](https://storage.examlex.com/TB2288/11ea7a3a_9f12_e965_a82d_e5f3a56722fa_TB2288_11.jpg)
CaSO3(s)
![<strong>What is the expression for K<sub>c</sub> for the following equilibrium? CaSO<sub>3</sub>(s) CaO(s)+ SO<sub>2</sub>(g)</strong> A)[CaO] [SO<sub>2</sub>] B)[SO<sub>2</sub>] C) D) E)](https://storage.examlex.com/TB2288/11ea7a3a_9f12_c252_a82d_f517eca7ddc6_TB2288_11.jpg)
CaO(s)+ SO2(g)
A)[CaO] [SO2]
B)[SO2]
C)
![<strong>What is the expression for K<sub>c</sub> for the following equilibrium? CaSO<sub>3</sub>(s) CaO(s)+ SO<sub>2</sub>(g)</strong> A)[CaO] [SO<sub>2</sub>] B)[SO<sub>2</sub>] C) D) E)](https://storage.examlex.com/TB2288/11ea7a3a_9f12_e963_a82d_dd47e3637ced_TB2288_11.jpg)
D)
![<strong>What is the expression for K<sub>c</sub> for the following equilibrium? CaSO<sub>3</sub>(s) CaO(s)+ SO<sub>2</sub>(g)</strong> A)[CaO] [SO<sub>2</sub>] B)[SO<sub>2</sub>] C) D) E)](https://storage.examlex.com/TB2288/11ea7a3a_9f12_e964_a82d_3d65c245f369_TB2288_11.jpg)
E)
![<strong>What is the expression for K<sub>c</sub> for the following equilibrium? CaSO<sub>3</sub>(s) CaO(s)+ SO<sub>2</sub>(g)</strong> A)[CaO] [SO<sub>2</sub>] B)[SO<sub>2</sub>] C) D) E)](https://storage.examlex.com/TB2288/11ea7a3a_9f12_e965_a82d_e5f3a56722fa_TB2288_11.jpg)

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
17
When gaseous carbon monoxide and hydrogen are combined in a sealed vessel and heated they will eventually form an equilbrium mixture of reactants and products according to the balanced chemical equilibrium below.
CO(g)+ 3H2(g)
CH4(g)+ H2O(g)
In one such reaction 3 moles of one reactant were combined with 1 mole of the other reactant in a fixed volume vessel and heated to 1200 K.Analysis of the reaction mixture at various times gave the results below.Which component of the reaction mixture is represented by curve B?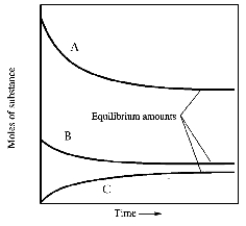
A)carbon monoxide
B)either methane or water
C)hydrogen
D)either hydrogen or carbon monoxide
E)not enough information to decide
CO(g)+ 3H2(g)

CH4(g)+ H2O(g)
In one such reaction 3 moles of one reactant were combined with 1 mole of the other reactant in a fixed volume vessel and heated to 1200 K.Analysis of the reaction mixture at various times gave the results below.Which component of the reaction mixture is represented by curve B?

A)carbon monoxide
B)either methane or water
C)hydrogen
D)either hydrogen or carbon monoxide
E)not enough information to decide

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
18
What balanced equation is the following equilibrium expression derived from? 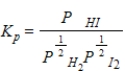
A) H2(g)+
H2(g)+  I2(g)
I2(g)  HI(g)
HI(g)
B)HI(g)
 H2(g)+
H2(g)+  I2(g)
I2(g)
C) H2(aq)+
H2(aq)+  I2(aq)
I2(aq)  HI(aq)
HI(aq)
D)HI(aq)
 H2(aq)+
H2(aq)+  I2(aq)
I2(aq)
E)2HI(g) H2(g)+ I2(g)
H2(g)+ I2(g)
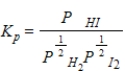
A)
 H2(g)+
H2(g)+  I2(g)
I2(g)  HI(g)
HI(g)B)HI(g)

 H2(g)+
H2(g)+  I2(g)
I2(g)C)
 H2(aq)+
H2(aq)+  I2(aq)
I2(aq)  HI(aq)
HI(aq)D)HI(aq)

 H2(aq)+
H2(aq)+  I2(aq)
I2(aq)E)2HI(g)
 H2(g)+ I2(g)
H2(g)+ I2(g)
Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
19
Which expression correctly describes the equilibrium constant Kc for the following reaction?
2C2H2(g)+ 5O2(g)
4CO2(g)+ 2H2O(g)
A)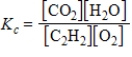
B)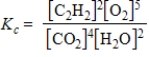
C)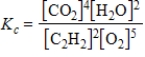
D)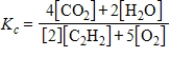
E)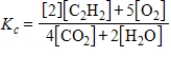
2C2H2(g)+ 5O2(g)

4CO2(g)+ 2H2O(g)
A)
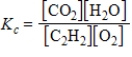
B)
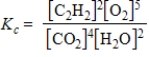
C)
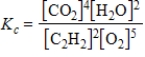
D)
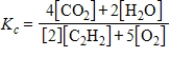
E)
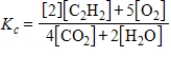

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
20
Apply the law of mass action to obtain the equilibrium-constant expression for the following reaction:
2X(g)+ Y(g)![<strong>Apply the law of mass action to obtain the equilibrium-constant expression for the following reaction: 2X(g)+ Y(g) 3W(g)+ V(g)</strong> A)[X]<sup>2</sup>[Y][W]<sup>3</sup>[V] B) C) D)](https://storage.examlex.com/TB2288/11ea7a3a_9f12_9b3e_a82d_53c3ee514e03_TB2288_11.jpg)
3W(g)+ V(g)
A)[X]2[Y][W]3[V]
B)![<strong>Apply the law of mass action to obtain the equilibrium-constant expression for the following reaction: 2X(g)+ Y(g) 3W(g)+ V(g)</strong> A)[X]<sup>2</sup>[Y][W]<sup>3</sup>[V] B) C) D)](https://storage.examlex.com/TB2288/11ea7a3a_9f12_9b3f_a82d_9d950e51fe87_TB2288_11.jpg)
C)![<strong>Apply the law of mass action to obtain the equilibrium-constant expression for the following reaction: 2X(g)+ Y(g) 3W(g)+ V(g)</strong> A)[X]<sup>2</sup>[Y][W]<sup>3</sup>[V] B) C) D)](https://storage.examlex.com/TB2288/11ea7a3a_9f12_c250_a82d_0bcb2356dd20_TB2288_11.jpg)
D)![<strong>Apply the law of mass action to obtain the equilibrium-constant expression for the following reaction: 2X(g)+ Y(g) 3W(g)+ V(g)</strong> A)[X]<sup>2</sup>[Y][W]<sup>3</sup>[V] B) C) D)](https://storage.examlex.com/TB2288/11ea7a3a_9f12_c251_a82d_8353ba93725b_TB2288_11.jpg)
2X(g)+ Y(g)
![<strong>Apply the law of mass action to obtain the equilibrium-constant expression for the following reaction: 2X(g)+ Y(g) 3W(g)+ V(g)</strong> A)[X]<sup>2</sup>[Y][W]<sup>3</sup>[V] B) C) D)](https://storage.examlex.com/TB2288/11ea7a3a_9f12_9b3e_a82d_53c3ee514e03_TB2288_11.jpg)
3W(g)+ V(g)
A)[X]2[Y][W]3[V]
B)
![<strong>Apply the law of mass action to obtain the equilibrium-constant expression for the following reaction: 2X(g)+ Y(g) 3W(g)+ V(g)</strong> A)[X]<sup>2</sup>[Y][W]<sup>3</sup>[V] B) C) D)](https://storage.examlex.com/TB2288/11ea7a3a_9f12_9b3f_a82d_9d950e51fe87_TB2288_11.jpg)
C)
![<strong>Apply the law of mass action to obtain the equilibrium-constant expression for the following reaction: 2X(g)+ Y(g) 3W(g)+ V(g)</strong> A)[X]<sup>2</sup>[Y][W]<sup>3</sup>[V] B) C) D)](https://storage.examlex.com/TB2288/11ea7a3a_9f12_c250_a82d_0bcb2356dd20_TB2288_11.jpg)
D)
![<strong>Apply the law of mass action to obtain the equilibrium-constant expression for the following reaction: 2X(g)+ Y(g) 3W(g)+ V(g)</strong> A)[X]<sup>2</sup>[Y][W]<sup>3</sup>[V] B) C) D)](https://storage.examlex.com/TB2288/11ea7a3a_9f12_c251_a82d_8353ba93725b_TB2288_11.jpg)

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
21
A sample of ammonia gas was allowed to come to equilibrium at 400 K.
2NH3(g)
N2(g)+ 3H2(g)
At equilibrium,it was found that the concentration of H2 was 0.0372 M,the concentration of N2 was 0.0124 M,and the concentration of NH3 was 0.175 M.What is Kp for this equilibrium? (R = 0.0821 L ∙ atm/(K ∙ mol))
A)4.28
B)2.85
C)1.70 × 10-2
D)1.95 × 10-8
E)2.26 × 10-2
2NH3(g)

N2(g)+ 3H2(g)
At equilibrium,it was found that the concentration of H2 was 0.0372 M,the concentration of N2 was 0.0124 M,and the concentration of NH3 was 0.175 M.What is Kp for this equilibrium? (R = 0.0821 L ∙ atm/(K ∙ mol))
A)4.28
B)2.85
C)1.70 × 10-2
D)1.95 × 10-8
E)2.26 × 10-2

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
22
Consider the following equilibrium:
1/2N2O4(g)
NO2(g); Kc = 3.3 at 100°C
For which of the following equilibria is Kc less than 3.3 at 100°C?
A)1/4N2O4(g) 1/2NO2(g)
1/2NO2(g)
B)N2O4(g) 2NO2(g)
2NO2(g)
C)4N2O4(g) 8NO2(g)
8NO2(g)
D)3N2O4(g) 6NO2(g)
6NO2(g)
E)2N2O4(g) 4NO2(g)
4NO2(g)
1/2N2O4(g)

NO2(g); Kc = 3.3 at 100°C
For which of the following equilibria is Kc less than 3.3 at 100°C?
A)1/4N2O4(g)
 1/2NO2(g)
1/2NO2(g)B)N2O4(g)
 2NO2(g)
2NO2(g)C)4N2O4(g)
 8NO2(g)
8NO2(g)D)3N2O4(g)
 6NO2(g)
6NO2(g)E)2N2O4(g)
 4NO2(g)
4NO2(g)
Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
23
If Kc = 0.133 for A2 + 2B  2AB,what is the value of Kc for the reaction
2AB,what is the value of Kc for the reaction
4AB
2A2 + 4B?
A)0.133
B)0.266
C)56.5
D)-0.133
E)3.76
 2AB,what is the value of Kc for the reaction
2AB,what is the value of Kc for the reaction4AB

2A2 + 4B?
A)0.133
B)0.266
C)56.5
D)-0.133
E)3.76

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
24
Given the equilibrium constants for the following reactions:
4Cu(s)+ O2(g)
2Cu2O(s); K1
2CuO(s)
Cu2O(s) + ½ O2(g); K2
What is K for the system
2Cu(s)+ O2(g)
2CuO(s)
Equivalent to?
A)(K2)2/(K1)
B)K1 × K2
C)(K1)(K2)1/2
D)(K2)½/(K1)
E)(K1)1/2/(K2)
4Cu(s)+ O2(g)

2Cu2O(s); K1
2CuO(s)

Cu2O(s) + ½ O2(g); K2
What is K for the system
2Cu(s)+ O2(g)

2CuO(s)
Equivalent to?
A)(K2)2/(K1)
B)K1 × K2
C)(K1)(K2)1/2
D)(K2)½/(K1)
E)(K1)1/2/(K2)

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
25
What is the Kc equilibrium-constant expression for the following equilibrium?
S8(s)+ 24F2(g)
8SF6(g)
A)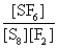
B)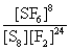
C)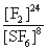
D)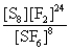
E)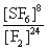
S8(s)+ 24F2(g)

8SF6(g)
A)
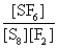
B)
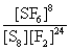
C)
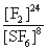
D)
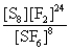
E)
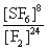

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
26
What is the Kp equilibrium-constant expression for the following equilibrium?
S8(s)+ 24F2(g)
8SF6(g)
A)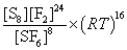
B)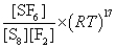
C)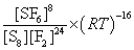
D)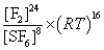
E)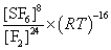
S8(s)+ 24F2(g)

8SF6(g)
A)
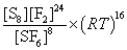
B)
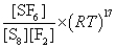
C)
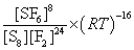
D)
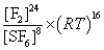
E)
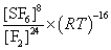

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
27
For the reaction 2NO(g)+ O2

2NO2(g)at 750°C,what is the relationship between Kc and Kp?
A)Kc = Kp
B)Kc = Kp × (RT)-1
C)Kc = Kp = 1.0
D)Kc = Kp × (RT)3/4
E)Kc = Kp × (RT)1

2NO2(g)at 750°C,what is the relationship between Kc and Kp?
A)Kc = Kp
B)Kc = Kp × (RT)-1
C)Kc = Kp = 1.0
D)Kc = Kp × (RT)3/4
E)Kc = Kp × (RT)1

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
28
What is the Kp equilibrium-constant expression for the following equilibrium?
Ti(s)+ 2Cl2(g)
TiCl4(l)
A)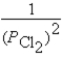
B)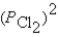
C)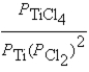
D)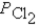
E)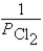
Ti(s)+ 2Cl2(g)

TiCl4(l)
A)
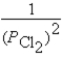
B)
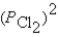
C)
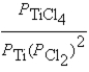
D)
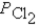
E)
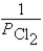

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
29
For which of the following reactions will the reactant experience the largest degree of decomposition upon reaching equilibrium at 500 K?
A)2NO2F(g) 2NO2(g)+ F2(g); Kp = 6.6 × 10-22
2NO2(g)+ F2(g); Kp = 6.6 × 10-22
B)2SO3(g) 2SO2(g)+ O2(g); Kp = 1.3 × 10-5
2SO2(g)+ O2(g); Kp = 1.3 × 10-5
C)2NOF(g) 2NO(g)+ F2(g); Kp = 1.2 × 10-26
2NO(g)+ F2(g); Kp = 1.2 × 10-26
D)2NOCl(g) 2NO(g)+ Cl2(g); Kp = 1.7 × 10-2
2NO(g)+ Cl2(g); Kp = 1.7 × 10-2
E)2NO2(g) 2NO(g)+ O2(g); Kp = 5.9 × 10-5
2NO(g)+ O2(g); Kp = 5.9 × 10-5
A)2NO2F(g)
 2NO2(g)+ F2(g); Kp = 6.6 × 10-22
2NO2(g)+ F2(g); Kp = 6.6 × 10-22B)2SO3(g)
 2SO2(g)+ O2(g); Kp = 1.3 × 10-5
2SO2(g)+ O2(g); Kp = 1.3 × 10-5C)2NOF(g)
 2NO(g)+ F2(g); Kp = 1.2 × 10-26
2NO(g)+ F2(g); Kp = 1.2 × 10-26D)2NOCl(g)
 2NO(g)+ Cl2(g); Kp = 1.7 × 10-2
2NO(g)+ Cl2(g); Kp = 1.7 × 10-2E)2NO2(g)
 2NO(g)+ O2(g); Kp = 5.9 × 10-5
2NO(g)+ O2(g); Kp = 5.9 × 10-5
Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
30
What is the Kc expression for the following equilibrium?
CuBr(s)![<strong>What is the K<sub>c</sub> expression for the following equilibrium? CuBr(s) Cu<sup>+</sup>(aq)+ Br<sup>-</sup>(aq)</strong> A)[Cu<sup>+</sup>] [Br<sup>-</sup>] B) C) D) E)](https://storage.examlex.com/TB2288/11ea7a3a_9f17_cbc4_a82d_77f2d5120763_TB2288_11.jpg)
Cu+(aq)+ Br-(aq)
A)[Cu+] [Br-]
B)![<strong>What is the K<sub>c</sub> expression for the following equilibrium? CuBr(s) Cu<sup>+</sup>(aq)+ Br<sup>-</sup>(aq)</strong> A)[Cu<sup>+</sup>] [Br<sup>-</sup>] B) C) D) E)](https://storage.examlex.com/TB2288/11ea7a3a_9f17_cbc5_a82d_5d77d00f7ac0_TB2288_11.jpg)
C)![<strong>What is the K<sub>c</sub> expression for the following equilibrium? CuBr(s) Cu<sup>+</sup>(aq)+ Br<sup>-</sup>(aq)</strong> A)[Cu<sup>+</sup>] [Br<sup>-</sup>] B) C) D) E)](https://storage.examlex.com/TB2288/11ea7a3a_9f17_f2d6_a82d_b73d082bc2a2_TB2288_11.jpg)
D)![<strong>What is the K<sub>c</sub> expression for the following equilibrium? CuBr(s) Cu<sup>+</sup>(aq)+ Br<sup>-</sup>(aq)</strong> A)[Cu<sup>+</sup>] [Br<sup>-</sup>] B) C) D) E)](https://storage.examlex.com/TB2288/11ea7a3a_9f17_f2d7_a82d_af7b92ec8077_TB2288_11.jpg)
E)![<strong>What is the K<sub>c</sub> expression for the following equilibrium? CuBr(s) Cu<sup>+</sup>(aq)+ Br<sup>-</sup>(aq)</strong> A)[Cu<sup>+</sup>] [Br<sup>-</sup>] B) C) D) E)](https://storage.examlex.com/TB2288/11ea7a3a_9f17_f2d8_a82d_0f6b6ddd0d66_TB2288_11.jpg)
CuBr(s)
![<strong>What is the K<sub>c</sub> expression for the following equilibrium? CuBr(s) Cu<sup>+</sup>(aq)+ Br<sup>-</sup>(aq)</strong> A)[Cu<sup>+</sup>] [Br<sup>-</sup>] B) C) D) E)](https://storage.examlex.com/TB2288/11ea7a3a_9f17_cbc4_a82d_77f2d5120763_TB2288_11.jpg)
Cu+(aq)+ Br-(aq)
A)[Cu+] [Br-]
B)
![<strong>What is the K<sub>c</sub> expression for the following equilibrium? CuBr(s) Cu<sup>+</sup>(aq)+ Br<sup>-</sup>(aq)</strong> A)[Cu<sup>+</sup>] [Br<sup>-</sup>] B) C) D) E)](https://storage.examlex.com/TB2288/11ea7a3a_9f17_cbc5_a82d_5d77d00f7ac0_TB2288_11.jpg)
C)
![<strong>What is the K<sub>c</sub> expression for the following equilibrium? CuBr(s) Cu<sup>+</sup>(aq)+ Br<sup>-</sup>(aq)</strong> A)[Cu<sup>+</sup>] [Br<sup>-</sup>] B) C) D) E)](https://storage.examlex.com/TB2288/11ea7a3a_9f17_f2d6_a82d_b73d082bc2a2_TB2288_11.jpg)
D)
![<strong>What is the K<sub>c</sub> expression for the following equilibrium? CuBr(s) Cu<sup>+</sup>(aq)+ Br<sup>-</sup>(aq)</strong> A)[Cu<sup>+</sup>] [Br<sup>-</sup>] B) C) D) E)](https://storage.examlex.com/TB2288/11ea7a3a_9f17_f2d7_a82d_af7b92ec8077_TB2288_11.jpg)
E)
![<strong>What is the K<sub>c</sub> expression for the following equilibrium? CuBr(s) Cu<sup>+</sup>(aq)+ Br<sup>-</sup>(aq)</strong> A)[Cu<sup>+</sup>] [Br<sup>-</sup>] B) C) D) E)](https://storage.examlex.com/TB2288/11ea7a3a_9f17_f2d8_a82d_0f6b6ddd0d66_TB2288_11.jpg)

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
31
At 298 K,the value of Kc for the reaction H2(g)+ Br2(g)  2HBr(g)is 2.0 × 1019.What is Kc for HBr(g)
2HBr(g)is 2.0 × 1019.What is Kc for HBr(g) 
1/2H2(g)+ 1/2Br2(g)?
A)4.0 × 10-38
B)5.0 × 10-20
C)1.0 × 1019
D)-2.0 × 1019
E)2.2 × 10-10
 2HBr(g)is 2.0 × 1019.What is Kc for HBr(g)
2HBr(g)is 2.0 × 1019.What is Kc for HBr(g) 
1/2H2(g)+ 1/2Br2(g)?
A)4.0 × 10-38
B)5.0 × 10-20
C)1.0 × 1019
D)-2.0 × 1019
E)2.2 × 10-10

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
32
Given the equilibrium constants for the equilibria,
22NH4+(aq)+ 2H2O(l)
2NH3(aq)+ 2H3O+(aq); Kc =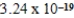
CH3COOH(aq)+ H2O(l)
CH3COO-(aq)+ H3O+(aq); Kc =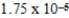
Determine Kc for the following equilibrium.
CH3COOH(aq)+ NH3(aq)
CH3COO-(aq)+ NH4+(aq)
A)3.08 × 104
B)3.25 × 10-5
C)9.96 × 10-15
D)1.00 × 1014
E)1.75 × 10-5
22NH4+(aq)+ 2H2O(l)

2NH3(aq)+ 2H3O+(aq); Kc =
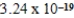
CH3COOH(aq)+ H2O(l)

CH3COO-(aq)+ H3O+(aq); Kc =
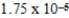
Determine Kc for the following equilibrium.
CH3COOH(aq)+ NH3(aq)

CH3COO-(aq)+ NH4+(aq)
A)3.08 × 104
B)3.25 × 10-5
C)9.96 × 10-15
D)1.00 × 1014
E)1.75 × 10-5

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
33
For which of the following values of the equilibrium constant does the reaction mixture contain mostly products?
A)100
B)101
C)109
D)10-1
E)10-9
A)100
B)101
C)109
D)10-1
E)10-9

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is always true for a reaction where Kc is 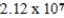 at 25°C?
at 25°C?
A)The reaction mixture contains mostly products at equilibrium.
B)The reaction mixture contains mostly reactants at equilibrium.
C)The rate of reaction is very fast.
D)There are approximately equal moles of reactants and products at equilibrium.
E)Both A and C.
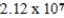 at 25°C?
at 25°C?A)The reaction mixture contains mostly products at equilibrium.
B)The reaction mixture contains mostly reactants at equilibrium.
C)The rate of reaction is very fast.
D)There are approximately equal moles of reactants and products at equilibrium.
E)Both A and C.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is true for a system whose equilibrium constant is much smaller than one?
A)The reaction mixture contains mostly reactants at equilibrium.
B)The reaction mixture contains mostly products at equilibrium.
C)The rate of reaction is very slow.
D)The moles of reactants and products are relatively similar at equilibrium.
E)Both A and C.
A)The reaction mixture contains mostly reactants at equilibrium.
B)The reaction mixture contains mostly products at equilibrium.
C)The rate of reaction is very slow.
D)The moles of reactants and products are relatively similar at equilibrium.
E)Both A and C.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
36
What is the Kc equilibrium-constant expression for the following equilibrium?
SrO(s)+ H2(g)
Sr(s)+ H2O(g)
A)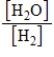
B)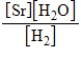
C)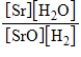
D)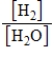
E)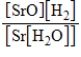
SrO(s)+ H2(g)

Sr(s)+ H2O(g)
A)
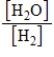
B)
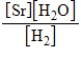
C)
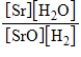
D)
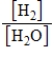
E)
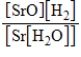

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
37
Consider the following reaction:
2HF(g)
H2(g)+ F2(g)(Kc = 1.00 × 10-2)
Given that 1.00 mol of HF(g),0.200 mol of H2(g),and 0.750 mol of F2(g)are mixed in a 5.00-L flask,determine the reaction quotient,Q.
A)Q = 0.0375
B)Q = 0.150
C)Q = 0.0300
D)Q = 1.95
E)none of these
2HF(g)

H2(g)+ F2(g)(Kc = 1.00 × 10-2)
Given that 1.00 mol of HF(g),0.200 mol of H2(g),and 0.750 mol of F2(g)are mixed in a 5.00-L flask,determine the reaction quotient,Q.
A)Q = 0.0375
B)Q = 0.150
C)Q = 0.0300
D)Q = 1.95
E)none of these

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
38
Consider the following equilibrium:
O2(g)+ 2F2(g)
2OF2(g); Kp = 2.3 × 10-15
Which of the following statements is true?
A)If the reaction mixture initially contains only OF2(g),then at equilibrium,the reaction mixture will consist of essentially only O2(g)and F2(g).
B)For this equilibrium,Kc = Kp.
C)If the reaction mixture initially contains only OF2(g),then the total pressure at equilibrium will be less than the total initial pressure.
D)If the reaction mixture initially contains only O2(g)and F2(g),then at equilibrium,the reaction mixture will consist of essentially only OF2(g).
E)If the reaction mixture initially contains only O2(g)and F2(g),then the total pressure at equilibrium will be greater than the total initial pressure.
O2(g)+ 2F2(g)

2OF2(g); Kp = 2.3 × 10-15
Which of the following statements is true?
A)If the reaction mixture initially contains only OF2(g),then at equilibrium,the reaction mixture will consist of essentially only O2(g)and F2(g).
B)For this equilibrium,Kc = Kp.
C)If the reaction mixture initially contains only OF2(g),then the total pressure at equilibrium will be less than the total initial pressure.
D)If the reaction mixture initially contains only O2(g)and F2(g),then at equilibrium,the reaction mixture will consist of essentially only OF2(g).
E)If the reaction mixture initially contains only O2(g)and F2(g),then the total pressure at equilibrium will be greater than the total initial pressure.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
39
For which of the following equilibria does Kc = Kp?
A)N2(g)+ 3H2(g) 2NH3(g)
2NH3(g)
B)CO(g)+ H2O(g) CO2(g)+ H2(g)
CO2(g)+ H2(g)
C)CO(g)+ 3H2(g) CH4(g)+ H2O(g)
CH4(g)+ H2O(g)
D)CaO(s)+ CO2(g) CaCO3(s)
CaCO3(s)
E)HBr(g) 1/2H2(g)+ 1/2Br2(l)
1/2H2(g)+ 1/2Br2(l)
A)N2(g)+ 3H2(g)
 2NH3(g)
2NH3(g)B)CO(g)+ H2O(g)
 CO2(g)+ H2(g)
CO2(g)+ H2(g)C)CO(g)+ 3H2(g)
 CH4(g)+ H2O(g)
CH4(g)+ H2O(g)D)CaO(s)+ CO2(g)
 CaCO3(s)
CaCO3(s)E)HBr(g)
 1/2H2(g)+ 1/2Br2(l)
1/2H2(g)+ 1/2Br2(l)
Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
40
Carbon tetrachloride may react with oxygen to produce chlorine and carbonyl chloride.
2CCl4(g)+ O2(g)
2COCl2(g)+ 2Cl2(g); Kc = 9.9 × 1051
What is Kc for the following equilibrium?
COCl2(g)+ Cl2(g)
CCl4(g)+ 1/2O2(g)
A)9.9 × 10-51
B)5.0 × 10-53
C)1.0 × 10-26
D)1.0 × 10-52
E)-9.9 × 1051
2CCl4(g)+ O2(g)

2COCl2(g)+ 2Cl2(g); Kc = 9.9 × 1051
What is Kc for the following equilibrium?
COCl2(g)+ Cl2(g)

CCl4(g)+ 1/2O2(g)
A)9.9 × 10-51
B)5.0 × 10-53
C)1.0 × 10-26
D)1.0 × 10-52
E)-9.9 × 1051

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
41
For the reaction 2H2S(g)  2H2(g)+ S2(g),Kc = 9.1 × 102 at 750 K.What will happen when 0.10 mol of H2S,1.0 mol of H2,and 1.5 mol of S2 are added to a 1.0-L container and the system is brought to 750 K?
2H2(g)+ S2(g),Kc = 9.1 × 102 at 750 K.What will happen when 0.10 mol of H2S,1.0 mol of H2,and 1.5 mol of S2 are added to a 1.0-L container and the system is brought to 750 K?
A)More S2 will be formed than H2.
B)More H2 will be formed than S2.
C)Nothing; the system is at equilibrium.
D)The amount of H2 formed will be half the amount of S2 formed.
E)More H2S will be formed.
 2H2(g)+ S2(g),Kc = 9.1 × 102 at 750 K.What will happen when 0.10 mol of H2S,1.0 mol of H2,and 1.5 mol of S2 are added to a 1.0-L container and the system is brought to 750 K?
2H2(g)+ S2(g),Kc = 9.1 × 102 at 750 K.What will happen when 0.10 mol of H2S,1.0 mol of H2,and 1.5 mol of S2 are added to a 1.0-L container and the system is brought to 750 K?A)More S2 will be formed than H2.
B)More H2 will be formed than S2.
C)Nothing; the system is at equilibrium.
D)The amount of H2 formed will be half the amount of S2 formed.
E)More H2S will be formed.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
42
Consider the following equilibrium:
C2H6(g)+ C5H12(g)
CH4(g)+ C6H14(g); Kp = 9.57 at 500 K
Suppose 11.6 g each of CH4,C2H6,C5H12,and C6H14 are placed in a 30.0-L reaction vessel at 500 K.Which of the following statements is correct?
A)Because Qc < Kc,more products will be formed.
B)Because Qc = 1,the system is at equilibrium.
C)Because Qc = 1,more products will be formed.
D)Because Qc = 1,more reactants will be formed.
E)Because Qc > Kc,more reactants will be formed.
C2H6(g)+ C5H12(g)

CH4(g)+ C6H14(g); Kp = 9.57 at 500 K
Suppose 11.6 g each of CH4,C2H6,C5H12,and C6H14 are placed in a 30.0-L reaction vessel at 500 K.Which of the following statements is correct?
A)Because Qc < Kc,more products will be formed.
B)Because Qc = 1,the system is at equilibrium.
C)Because Qc = 1,more products will be formed.
D)Because Qc = 1,more reactants will be formed.
E)Because Qc > Kc,more reactants will be formed.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
43
Consider the following equilibrium:
2NO(g)+ 3F2(g)
2NOF3(g)
Suppose 0.20 mol of NO and 0.30 mol of F2 are added to a 5.0-L container.If x mol of NOF3 is present at equilibrium,what is the equilibrium concentration of NO?
A)0.20x
B)0.05 - 0.50x
C)0.20 - 2x
D)0.04 - 0.20x
E)0.20 - x
2NO(g)+ 3F2(g)

2NOF3(g)
Suppose 0.20 mol of NO and 0.30 mol of F2 are added to a 5.0-L container.If x mol of NOF3 is present at equilibrium,what is the equilibrium concentration of NO?
A)0.20x
B)0.05 - 0.50x
C)0.20 - 2x
D)0.04 - 0.20x
E)0.20 - x

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
44
For the equilibrium PCl5(g)  PCl3(g)+ Cl2(g),Kc = 2.0 × 101 at 240°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl3(g)is 0.27 M,what is the equilibrium concentration of PCl5(g)?
PCl3(g)+ Cl2(g),Kc = 2.0 × 101 at 240°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl3(g)is 0.27 M,what is the equilibrium concentration of PCl5(g)?
A)0.54 M
B)0.13 M
C)0.013 M
D)0.0036 M
E)8.6 M
 PCl3(g)+ Cl2(g),Kc = 2.0 × 101 at 240°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl3(g)is 0.27 M,what is the equilibrium concentration of PCl5(g)?
PCl3(g)+ Cl2(g),Kc = 2.0 × 101 at 240°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl3(g)is 0.27 M,what is the equilibrium concentration of PCl5(g)?A)0.54 M
B)0.13 M
C)0.013 M
D)0.0036 M
E)8.6 M

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
45
Consider the following equilibrium:
4NH3(g)+ 3O2(g)
2N2(g)+ 6H2O(g)
Suppose 0.30 mol of NH3 and 0.40 mol of oxygen are added to a 5.0-L container.If x mol of water is present at equilibrium,how many moles of ammonia will remain at equilibrium?
A)0.30 -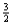 x
x
B)0.30 - x
C)0.30 -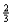 x
x
D)0.40 -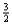 x
x
E)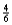 x
x
4NH3(g)+ 3O2(g)

2N2(g)+ 6H2O(g)
Suppose 0.30 mol of NH3 and 0.40 mol of oxygen are added to a 5.0-L container.If x mol of water is present at equilibrium,how many moles of ammonia will remain at equilibrium?
A)0.30 -
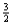 x
xB)0.30 - x
C)0.30 -
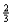 x
xD)0.40 -
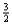 x
xE)
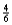 x
x
Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
46
At 700 K,Kp for the following equilibrium is 5.6 × 10-3.
2HgO(s)
2Hg(l)+ O2(g)
Suppose 72.2 g of mercury(II)oxide is placed in a sealed 1.00-L vessel at 700 K.What is the partial pressure of oxygen gas at equilibrium? (R = 0.0821 L ∙ atm/(K ∙ mol))
A)0.074 atm
B)0.0056 atm
C)19 atm
D)57 atm
E)9.5 atm
2HgO(s)

2Hg(l)+ O2(g)
Suppose 72.2 g of mercury(II)oxide is placed in a sealed 1.00-L vessel at 700 K.What is the partial pressure of oxygen gas at equilibrium? (R = 0.0821 L ∙ atm/(K ∙ mol))
A)0.074 atm
B)0.0056 atm
C)19 atm
D)57 atm
E)9.5 atm

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
47
In an experiment,0.30 mol H2 and 0.30 mol I2 are mixed in a 1.00-L container,and the reaction forms HI.If Kc = 49.for this reaction,what is the equilibrium concentration of HI?
I2(g)+ H2(g)
2HI(g)
A)0.58 M
B)0.53 M
C)0.47 M
D)0.075 M
E)0.040 M
I2(g)+ H2(g)

2HI(g)
A)0.58 M
B)0.53 M
C)0.47 M
D)0.075 M
E)0.040 M

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
48
Consider the following equilibrium:
2NO(g)+ 3F2(g)
2NOF3(g)
Suppose 0.20 mol of NO and 0.30 mol of F2 are added to a 5.0-L container.If x mol of NOF3 is present at equilibrium,how many moles of fluorine are present at equilibrium?
A)0.30 - x
B)0.30 - 2x
C)0.06 - 0.20x
D)0.30 -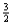 x
x
E)0.20 - x
2NO(g)+ 3F2(g)

2NOF3(g)
Suppose 0.20 mol of NO and 0.30 mol of F2 are added to a 5.0-L container.If x mol of NOF3 is present at equilibrium,how many moles of fluorine are present at equilibrium?
A)0.30 - x
B)0.30 - 2x
C)0.06 - 0.20x
D)0.30 -
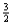 x
xE)0.20 - x

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
49
The reaction quotient for a system is 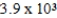 .If the equilibrium constant for the system is
.If the equilibrium constant for the system is 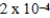
,what will happen as the reaction mixture approaches equilibrium?
A)The equilibrium constant will increase until it equals the reaction quotient.
B)There will be a net gain in both product(s)and reactant(s).
C)There will be a net gain in product(s).
D)There will be a net gain in reactant(s).
E)The equilibrium constant will decrease until it equals the reaction quotient.
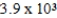 .If the equilibrium constant for the system is
.If the equilibrium constant for the system is 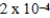
,what will happen as the reaction mixture approaches equilibrium?
A)The equilibrium constant will increase until it equals the reaction quotient.
B)There will be a net gain in both product(s)and reactant(s).
C)There will be a net gain in product(s).
D)There will be a net gain in reactant(s).
E)The equilibrium constant will decrease until it equals the reaction quotient.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
50
At 800 K,Kc for the following equilibrium is 4.2 × 10-3.
2HgO(s)
2Hg(l)+ O2(g)
Suppose 82.3 g of mercury (II)oxide is placed in a sealed 2.50-L vessel at 800 K.What is the partial pressure of oxygen gas at equilibrium? (R = 0.0821 L ∙ atm/(K ∙ mol))
A)8.7 atm
B)4.3 atm
C)0.27 atm
D)0.0042 atm
E)22 atm
2HgO(s)

2Hg(l)+ O2(g)
Suppose 82.3 g of mercury (II)oxide is placed in a sealed 2.50-L vessel at 800 K.What is the partial pressure of oxygen gas at equilibrium? (R = 0.0821 L ∙ atm/(K ∙ mol))
A)8.7 atm
B)4.3 atm
C)0.27 atm
D)0.0042 atm
E)22 atm

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
51
CS2(g)+ 3Cl2(g) ![<strong>CS<sub>2</sub>(g)+ 3Cl<sub>2</sub>(g) CCl<sub>4</sub>(g)+ S<sub>2</sub>Cl<sub>2</sub>(g) At a given temperature,the reaction above is at equilibrium when [CS<sub>2</sub>] = 0.050 M,[Cl<sub>2</sub>] = 0.25 M,[CCl<sub>4</sub>] = 0.15 M,and [S<sub>2</sub>Cl<sub>2</sub>] = 0.35 M.What will be the direction of the reaction when the reactants and products have the following concentrations: CS<sub>2</sub> = 0.14 M,Cl<sub>2</sub> = 0.20 M,CCl<sub>4</sub> = 0.28 M,and S<sub>2</sub>Cl<sub>2</sub> = 0.28 M?</strong> A)to the left B)to the right C)no change D)cannot predict unless we know the temperature E)cannot predict unless we know whether the reaction is endothermic or exothermic](https://storage.examlex.com/TB2288/11ea7a3a_9f19_a09e_a82d_05e13d793f7a_TB2288_11.jpg) CCl4(g)+ S2Cl2(g)
CCl4(g)+ S2Cl2(g)
At a given temperature,the reaction above is at equilibrium when [CS2] = 0.050 M,[Cl2] = 0.25 M,[CCl4] = 0.15 M,and [S2Cl2] = 0.35 M.What will be the direction of the reaction when the reactants and products have the following concentrations: CS2 = 0.14 M,Cl2 = 0.20 M,CCl4 = 0.28 M,and S2Cl2 = 0.28 M?
A)to the left
B)to the right
C)no change
D)cannot predict unless we know the temperature
E)cannot predict unless we know whether the reaction is endothermic or exothermic
![<strong>CS<sub>2</sub>(g)+ 3Cl<sub>2</sub>(g) CCl<sub>4</sub>(g)+ S<sub>2</sub>Cl<sub>2</sub>(g) At a given temperature,the reaction above is at equilibrium when [CS<sub>2</sub>] = 0.050 M,[Cl<sub>2</sub>] = 0.25 M,[CCl<sub>4</sub>] = 0.15 M,and [S<sub>2</sub>Cl<sub>2</sub>] = 0.35 M.What will be the direction of the reaction when the reactants and products have the following concentrations: CS<sub>2</sub> = 0.14 M,Cl<sub>2</sub> = 0.20 M,CCl<sub>4</sub> = 0.28 M,and S<sub>2</sub>Cl<sub>2</sub> = 0.28 M?</strong> A)to the left B)to the right C)no change D)cannot predict unless we know the temperature E)cannot predict unless we know whether the reaction is endothermic or exothermic](https://storage.examlex.com/TB2288/11ea7a3a_9f19_a09e_a82d_05e13d793f7a_TB2288_11.jpg) CCl4(g)+ S2Cl2(g)
CCl4(g)+ S2Cl2(g)At a given temperature,the reaction above is at equilibrium when [CS2] = 0.050 M,[Cl2] = 0.25 M,[CCl4] = 0.15 M,and [S2Cl2] = 0.35 M.What will be the direction of the reaction when the reactants and products have the following concentrations: CS2 = 0.14 M,Cl2 = 0.20 M,CCl4 = 0.28 M,and S2Cl2 = 0.28 M?
A)to the left
B)to the right
C)no change
D)cannot predict unless we know the temperature
E)cannot predict unless we know whether the reaction is endothermic or exothermic

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
52
Hydrogen iodide undergoes decomposition according to the equation
2HI(g)
H2(g)+ I2(g)
The equilibrium constant Kp at 500 K for this equilibrium is 0.060.Suppose 0.811 mol of HI is placed in a 1.50-L container at 500 K.What is the equilibrium concentration of H2(g)?
(R = 0.0821 L ∙ atm/(K ∙ mol))
A)0.21 M
B)0.13 M
C)4.3 M
D)0.039 M
E)0.1 M
2HI(g)

H2(g)+ I2(g)
The equilibrium constant Kp at 500 K for this equilibrium is 0.060.Suppose 0.811 mol of HI is placed in a 1.50-L container at 500 K.What is the equilibrium concentration of H2(g)?
(R = 0.0821 L ∙ atm/(K ∙ mol))
A)0.21 M
B)0.13 M
C)4.3 M
D)0.039 M
E)0.1 M

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
53
Consider the following equilibrium:
C2H6(g)+ C5H12(g)
CH4(g)+ C6H14(g); Kp = 9.57 at 500 K
Suppose 13.3 g each of CH4,C2H6,C5H12,and C6H14 are placed in a 50.0-L reaction vessel at 500 K.What is the value of Qp?
A)1
B)0.104
C)1.56
D)0.637
E)9.57
C2H6(g)+ C5H12(g)

CH4(g)+ C6H14(g); Kp = 9.57 at 500 K
Suppose 13.3 g each of CH4,C2H6,C5H12,and C6H14 are placed in a 50.0-L reaction vessel at 500 K.What is the value of Qp?
A)1
B)0.104
C)1.56
D)0.637
E)9.57

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
54
For the equilibrium N2O4(g)  2NO2(g),at 298 K,Kp = 0.15.For this reaction system,it is found that the partial pressure of N2O4 is 3.5 × 10-2 atm at equilibrium.What is the partial pressure of NO2 at equilibrium? (R = 0.0821 L ∙ atm/(K ∙ mol))
2NO2(g),at 298 K,Kp = 0.15.For this reaction system,it is found that the partial pressure of N2O4 is 3.5 × 10-2 atm at equilibrium.What is the partial pressure of NO2 at equilibrium? (R = 0.0821 L ∙ atm/(K ∙ mol))
A)4.8 atm
B)23 atm
C)0.0018 atm
D)0.0052 atm
E)0.072 atm
 2NO2(g),at 298 K,Kp = 0.15.For this reaction system,it is found that the partial pressure of N2O4 is 3.5 × 10-2 atm at equilibrium.What is the partial pressure of NO2 at equilibrium? (R = 0.0821 L ∙ atm/(K ∙ mol))
2NO2(g),at 298 K,Kp = 0.15.For this reaction system,it is found that the partial pressure of N2O4 is 3.5 × 10-2 atm at equilibrium.What is the partial pressure of NO2 at equilibrium? (R = 0.0821 L ∙ atm/(K ∙ mol))A)4.8 atm
B)23 atm
C)0.0018 atm
D)0.0052 atm
E)0.072 atm

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
55
For the reaction 2HI(g)  H2(g)+ I2(g),Kc = 0.290 at 400 K.If the initial concentrations of HI,H2,and I2 are all 1.50 × 10-3 M at 400 K,which one of the following statements is correct?
H2(g)+ I2(g),Kc = 0.290 at 400 K.If the initial concentrations of HI,H2,and I2 are all 1.50 × 10-3 M at 400 K,which one of the following statements is correct?
A)The concentrations of HI and I2 will increase as the system is approaching equilibrium.
B)The concentrations of H2 and I2 will increase as the system is approaching equilibrium.
C)The system is at equilibrium.
D)The concentrations of H2 and HI will decrease as the system is approaching equilibrium.
E)The concentration of HI will increase as the system is approaching equilibrium.
 H2(g)+ I2(g),Kc = 0.290 at 400 K.If the initial concentrations of HI,H2,and I2 are all 1.50 × 10-3 M at 400 K,which one of the following statements is correct?
H2(g)+ I2(g),Kc = 0.290 at 400 K.If the initial concentrations of HI,H2,and I2 are all 1.50 × 10-3 M at 400 K,which one of the following statements is correct?A)The concentrations of HI and I2 will increase as the system is approaching equilibrium.
B)The concentrations of H2 and I2 will increase as the system is approaching equilibrium.
C)The system is at equilibrium.
D)The concentrations of H2 and HI will decrease as the system is approaching equilibrium.
E)The concentration of HI will increase as the system is approaching equilibrium.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
56
Hydrogen iodide undergoes decomposition according to the equation
2HI(g)
H2(g)+ I2(g)
The equilibrium constant Kc at 425°C for this system is 0.018.If 1.0 mol each of H2,I2,and HI were placed together in a 1-L container at 425°C,then
A)because of reaction,the total number of molecules would increase.
B)the concentration of HI would decrease.
C)because of reaction,the total number of molecules would decrease.
D)the value of K would increase to 1.0.
E)the concentration of H2 would decrease.
2HI(g)

H2(g)+ I2(g)
The equilibrium constant Kc at 425°C for this system is 0.018.If 1.0 mol each of H2,I2,and HI were placed together in a 1-L container at 425°C,then
A)because of reaction,the total number of molecules would increase.
B)the concentration of HI would decrease.
C)because of reaction,the total number of molecules would decrease.
D)the value of K would increase to 1.0.
E)the concentration of H2 would decrease.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
57
For the equilibrium PCl5(g)  PCl3(g)+ Cl2(g),Kc = 4.0 at 228°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl5(g)is 0.26 M,what is the equilibrium concentration of PCl3?
PCl3(g)+ Cl2(g),Kc = 4.0 at 228°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl5(g)is 0.26 M,what is the equilibrium concentration of PCl3?
A)0.11 M
B)0.38 M
C)0.23 M
D)0.95 M
E)0.013 M
 PCl3(g)+ Cl2(g),Kc = 4.0 at 228°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl5(g)is 0.26 M,what is the equilibrium concentration of PCl3?
PCl3(g)+ Cl2(g),Kc = 4.0 at 228°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl5(g)is 0.26 M,what is the equilibrium concentration of PCl3?A)0.11 M
B)0.38 M
C)0.23 M
D)0.95 M
E)0.013 M

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
58
What is the reaction quotient (Q)for the equilibrium
TlSCN(s)
Tl+(aq)+ SCN-(aq)
When 0.1837 L of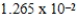
M Tl+ is combined with 0.1335 L of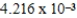
M SCN- in the presence of an excess of TlSCN(s)?
A)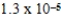
B)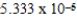
C)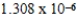
D)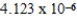
E)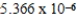
TlSCN(s)

Tl+(aq)+ SCN-(aq)
When 0.1837 L of
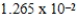
M Tl+ is combined with 0.1335 L of
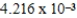
M SCN- in the presence of an excess of TlSCN(s)?
A)
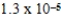
B)
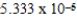
C)
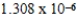
D)
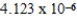
E)
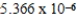

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
59
Consider the following equilibrium.
4NH3(g)+ 3O2(g)
2N2(g)+ 6H2O(g)
Suppose 0.30 mol of NH3 and 0.40 mol of oxygen are added to a 5.0-L container.If x mol of water is present at equilibrium,what is the equilibrium concentration of oxygen?
A)0.40 - 0.50x
B)0.30 - 0.50x
C)0.08 - 0.10x
D)0.06 - 0.13x
E)0.40 -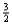 x
x
4NH3(g)+ 3O2(g)

2N2(g)+ 6H2O(g)
Suppose 0.30 mol of NH3 and 0.40 mol of oxygen are added to a 5.0-L container.If x mol of water is present at equilibrium,what is the equilibrium concentration of oxygen?
A)0.40 - 0.50x
B)0.30 - 0.50x
C)0.08 - 0.10x
D)0.06 - 0.13x
E)0.40 -
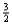 x
x
Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
60
The equilibrium constant for the reaction H2(g)+ I2(g) ![<strong>The equilibrium constant for the reaction H<sub>2</sub>(g)+ I<sub>2</sub>(g) 2HI(g)is 62.5 at 800 K.What is the equilibrium concentration of I<sub>2</sub> if at equilibrium [HI] = 0.21 M and [H<sub>2</sub>] = 0.13 M?</strong> A)5.4 × 10<sup>-3</sup> M B)0.29 M C)5.2 × 10<sup>-2</sup> M D)0.21 M E)2.6 × 10<sup>-2</sup> M](https://storage.examlex.com/TB2288/11ea7a3a_9f1a_63f4_a82d_1b3eb2b8c365_TB2288_11.jpg) 2HI(g)is 62.5 at 800 K.What is the equilibrium concentration of I2 if at equilibrium [HI] = 0.21 M and [H2] = 0.13 M?
2HI(g)is 62.5 at 800 K.What is the equilibrium concentration of I2 if at equilibrium [HI] = 0.21 M and [H2] = 0.13 M?
A)5.4 × 10-3 M
B)0.29 M
C)5.2 × 10-2 M
D)0.21 M
E)2.6 × 10-2 M
![<strong>The equilibrium constant for the reaction H<sub>2</sub>(g)+ I<sub>2</sub>(g) 2HI(g)is 62.5 at 800 K.What is the equilibrium concentration of I<sub>2</sub> if at equilibrium [HI] = 0.21 M and [H<sub>2</sub>] = 0.13 M?</strong> A)5.4 × 10<sup>-3</sup> M B)0.29 M C)5.2 × 10<sup>-2</sup> M D)0.21 M E)2.6 × 10<sup>-2</sup> M](https://storage.examlex.com/TB2288/11ea7a3a_9f1a_63f4_a82d_1b3eb2b8c365_TB2288_11.jpg) 2HI(g)is 62.5 at 800 K.What is the equilibrium concentration of I2 if at equilibrium [HI] = 0.21 M and [H2] = 0.13 M?
2HI(g)is 62.5 at 800 K.What is the equilibrium concentration of I2 if at equilibrium [HI] = 0.21 M and [H2] = 0.13 M?A)5.4 × 10-3 M
B)0.29 M
C)5.2 × 10-2 M
D)0.21 M
E)2.6 × 10-2 M

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
61
For which of the following systems at equilibrium and at constant temperature will increasing the volume have no effect on the equilibrium?
A)C(s)+ CO2(g) 2CO(g)
2CO(g)
B)SO2Cl2(g) SO2(g)+ Cl2(g)
SO2(g)+ Cl2(g)
C)COCl2(g) CO(g)+ Cl2(g)
CO(g)+ Cl2(g)
D)I2(g) 2I(g)
2I(g)
E)CO(g)+ H2O(g) CO2(g)+ H2(g)
CO2(g)+ H2(g)
A)C(s)+ CO2(g)
 2CO(g)
2CO(g)B)SO2Cl2(g)
 SO2(g)+ Cl2(g)
SO2(g)+ Cl2(g)C)COCl2(g)
 CO(g)+ Cl2(g)
CO(g)+ Cl2(g)D)I2(g)
 2I(g)
2I(g)E)CO(g)+ H2O(g)
 CO2(g)+ H2(g)
CO2(g)+ H2(g)
Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
62
At 550 K,Kp = 7.7 × 102 for the following equilibrium.
SO2(g)+ NO2(g)
SO3(g)+ NO(g)
If 0.279 mol each of SO3 and NO are placed in a 4.00-L container at 550 K,what is the concentration of SO3 at equilibrium? (R = 0.0821 L · atm/(K · mol))
A)3 M
B)0.067 M
C)0.069 M
D)17 M
E)0.017 M
SO2(g)+ NO2(g)

SO3(g)+ NO(g)
If 0.279 mol each of SO3 and NO are placed in a 4.00-L container at 550 K,what is the concentration of SO3 at equilibrium? (R = 0.0821 L · atm/(K · mol))
A)3 M
B)0.067 M
C)0.069 M
D)17 M
E)0.017 M

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
63
In which of the following reactions does an instantaneous increase in the volume of the reaction vessel favor formation of the products?
A)PCl5(g) PCl3(g)+ Cl2(g)
PCl3(g)+ Cl2(g)
B)N2(g)+ 3H2(g) 2NH3(g)
2NH3(g)
C)H2(g)+ I2(g) 2HI(g)
2HI(g)
D)MgO(s)+ CO2(g) MgCO3(s)
MgCO3(s)
E)N2(g)+ O2(g) 2NO(g)
2NO(g)
A)PCl5(g)
 PCl3(g)+ Cl2(g)
PCl3(g)+ Cl2(g)B)N2(g)+ 3H2(g)
 2NH3(g)
2NH3(g)C)H2(g)+ I2(g)
 2HI(g)
2HI(g)D)MgO(s)+ CO2(g)
 MgCO3(s)
MgCO3(s)E)N2(g)+ O2(g)
 2NO(g)
2NO(g)
Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following equilibria would not be affected by pressure changes at constant temperature?
A)CO2(g)+ H2(g) CO(g)+ H2O(g)
CO(g)+ H2O(g)
B)CO(g)+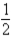 O2(g)
O2(g)  CO2(g)
CO2(g)
C)2Hg(l)+ O2(g) 2HgO(s)
2HgO(s)
D)2H2(g)+ O2(g) 2H2O(l)
2H2O(l)
E)CaCO3(s) CaO(s)+ CO2(g)
CaO(s)+ CO2(g)
A)CO2(g)+ H2(g)
 CO(g)+ H2O(g)
CO(g)+ H2O(g)B)CO(g)+
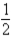 O2(g)
O2(g)  CO2(g)
CO2(g)C)2Hg(l)+ O2(g)
 2HgO(s)
2HgO(s)D)2H2(g)+ O2(g)
 2H2O(l)
2H2O(l)E)CaCO3(s)
 CaO(s)+ CO2(g)
CaO(s)+ CO2(g)
Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
65
For which of the following systems at equilibrium and at constant temperature will decreasing the volume cause the equilibrium to shift to the right?
A)NH4Cl(s) NH3(g)+ HCl(g)
NH3(g)+ HCl(g)
B)2NO2(g) 2NO(g)+ O2(g)
2NO(g)+ O2(g)
C)H2(g)+ Cl2(g) 2HCl(g)
2HCl(g)
D)N2(g)+ 3H2(g) 2NH3(g)
2NH3(g)
E)2H2O(g) 2H2(g)+ O2(g)
2H2(g)+ O2(g)
A)NH4Cl(s)
 NH3(g)+ HCl(g)
NH3(g)+ HCl(g)B)2NO2(g)
 2NO(g)+ O2(g)
2NO(g)+ O2(g)C)H2(g)+ Cl2(g)
 2HCl(g)
2HCl(g)D)N2(g)+ 3H2(g)
 2NH3(g)
2NH3(g)E)2H2O(g)
 2H2(g)+ O2(g)
2H2(g)+ O2(g)
Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following,when added to an equilibrium mixture represented by the equlibrium below,will not alter the composition of the original equilibrium mixture?
Mg(OH)2(s)
Mg2+(aq)+ 2OH-(aq)
A)Addition of Mg(NO3)2(s)to the equilibrium mixture.
B)Addition of Fe(NO3)3(aq)from the equilibrium mixture.
C)Addition of Mg(OH)2(s)to the equilibrium mixture.
D)Addition of HCl(aq)to the equilibrium mixture.
E)Addition of NaOH(s)to the equilibrium mixture.
Mg(OH)2(s)

Mg2+(aq)+ 2OH-(aq)
A)Addition of Mg(NO3)2(s)to the equilibrium mixture.
B)Addition of Fe(NO3)3(aq)from the equilibrium mixture.
C)Addition of Mg(OH)2(s)to the equilibrium mixture.
D)Addition of HCl(aq)to the equilibrium mixture.
E)Addition of NaOH(s)to the equilibrium mixture.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
67
Consider the following equilibrium:
NH4Cl(s)
NH3(g)+ HCl(g)
Suppose a vessel containing NH4Cl(s),NH3(g)and HCl(g)is at equilibrium.If the volume of the vessel is instantaneously doubled while keeping the temperature constant,when a new equilibrium is reached,which of the following statements is incorrect?
A)The value of Kp remains unchanged.
B)The number of moles of NH4Cl decreases.
C)The total pressure is halved.
D)The partial pressures of NH3 and HCl in the vessel remain unchanged.
E)The amount of NH3 and HCl doubles.
NH4Cl(s)

NH3(g)+ HCl(g)
Suppose a vessel containing NH4Cl(s),NH3(g)and HCl(g)is at equilibrium.If the volume of the vessel is instantaneously doubled while keeping the temperature constant,when a new equilibrium is reached,which of the following statements is incorrect?
A)The value of Kp remains unchanged.
B)The number of moles of NH4Cl decreases.
C)The total pressure is halved.
D)The partial pressures of NH3 and HCl in the vessel remain unchanged.
E)The amount of NH3 and HCl doubles.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
68
Ammonia is prepared industrially by the reaction
N2(g)+ 3H2(g)
2NH3(g)
For the reaction,ΔH° = -92.2 kJ and K (at 25°C)= 4.0 × 108.When the temperature of the reaction is increased to 500°C,which of the following statements is true?
A)At equilibrium,more NH3 is present at 500°C than at 25°C.
B)The reaction of N2 with H2 to form ammonia is endothermic.
C)K for the reaction will be larger at 500°C than at 25°C.
D)Product formation (at equilibrium)is less favored as the temperature is raised.
E)None of the above statements is true.
N2(g)+ 3H2(g)

2NH3(g)
For the reaction,ΔH° = -92.2 kJ and K (at 25°C)= 4.0 × 108.When the temperature of the reaction is increased to 500°C,which of the following statements is true?
A)At equilibrium,more NH3 is present at 500°C than at 25°C.
B)The reaction of N2 with H2 to form ammonia is endothermic.
C)K for the reaction will be larger at 500°C than at 25°C.
D)Product formation (at equilibrium)is less favored as the temperature is raised.
E)None of the above statements is true.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
69
When cobalt chloride is added to pure water,the Co2+ ions hydrate.The hydrated form then reacts with the Cl- ions to set up the equilibrium shown here:
Co(H2O)62+ + 4Cl-
CoCl42- + 6H2O
(pink)
(blue)
Which statement accurately describes the change that the system will undergo if water is added?
A)The color will become more blue.
B)The equilibrium will shift to the right.
C)More water will be produced.
D)More chloride ions will be produced.
E)There will be less of the hydrated cobalt ion at the new equilibrium position.
Co(H2O)62+ + 4Cl-

CoCl42- + 6H2O
(pink)
(blue)
Which statement accurately describes the change that the system will undergo if water is added?
A)The color will become more blue.
B)The equilibrium will shift to the right.
C)More water will be produced.
D)More chloride ions will be produced.
E)There will be less of the hydrated cobalt ion at the new equilibrium position.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
70
Consider the reaction represented by the equation N2(g)+ 3H2(g)  2NH3(g).What happens to the equilibrium position when an inert gas is added to this system (as represented above)at equilibrium?
2NH3(g).What happens to the equilibrium position when an inert gas is added to this system (as represented above)at equilibrium?
A)If the container is rigid,the equilibrium position shifts.If the container is fitted with a movable piston,nothing happens to the equilibrium position.
B)If the container is rigid,nothing happens to the equilibrium position.If the container is fitted with a movable piston,the equilibrium position shifts.
C)Nothing happens to the equilibrium position no matter what the container is like.
D)The equilibrium position shifts no matter what the container is like.
E)The value of the equilibrium constant must be known to answer this question.
 2NH3(g).What happens to the equilibrium position when an inert gas is added to this system (as represented above)at equilibrium?
2NH3(g).What happens to the equilibrium position when an inert gas is added to this system (as represented above)at equilibrium?A)If the container is rigid,the equilibrium position shifts.If the container is fitted with a movable piston,nothing happens to the equilibrium position.
B)If the container is rigid,nothing happens to the equilibrium position.If the container is fitted with a movable piston,the equilibrium position shifts.
C)Nothing happens to the equilibrium position no matter what the container is like.
D)The equilibrium position shifts no matter what the container is like.
E)The value of the equilibrium constant must be known to answer this question.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
71
What effect will spraying liquid water into the equilibrium given below have if NH3 is far more soluble in water than is N2 or H2?
N2(g)+ 3H2(g)
2NH3(g)
A)More NH3(g)will form.
B)More H2(g)will form.
C)Less NH3(g)will form.
D)This will not affect the system.
E)More N2(g)will form.
N2(g)+ 3H2(g)

2NH3(g)
A)More NH3(g)will form.
B)More H2(g)will form.
C)Less NH3(g)will form.
D)This will not affect the system.
E)More N2(g)will form.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following equilibria would be affected by volume changes at constant temperature?
A)HCl(aq)+ NaOH(aq) H2O(l)+ NaCl(aq)
H2O(l)+ NaCl(aq)
B)C2H4(g)+ H2(g) C2H6(g)
C2H6(g)
C)2HCl(g) H2(g)+ Cl2(g)
H2(g)+ Cl2(g)
D)SO3(g)+ NO(g) NO2(g)+ SO2(g)
NO2(g)+ SO2(g)
E)2HF(g) H2(g)+ F2(g)
H2(g)+ F2(g)
A)HCl(aq)+ NaOH(aq)
 H2O(l)+ NaCl(aq)
H2O(l)+ NaCl(aq)B)C2H4(g)+ H2(g)
 C2H6(g)
C2H6(g)C)2HCl(g)
 H2(g)+ Cl2(g)
H2(g)+ Cl2(g)D)SO3(g)+ NO(g)
 NO2(g)+ SO2(g)
NO2(g)+ SO2(g)E)2HF(g)
 H2(g)+ F2(g)
H2(g)+ F2(g)
Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
73
Consider the following equilibrium:
CO2(g)+ H2(g)
CO(g)+ H2O(g); Kc = 1.6 at 1260 K
Suppose 0.037 mol CO2 and 0.020 mol H2 are placed in a 3.50-L vessel at 1260 K.What is the equilibrium partial pressure of CO(g)? (R = 0.0821 L ∙ atm/(K ∙ mol))
A)4.1 atm
B)0.5 atm
C)1.6 atm
D)1.1 atm
E)0.59 atm
CO2(g)+ H2(g)

CO(g)+ H2O(g); Kc = 1.6 at 1260 K
Suppose 0.037 mol CO2 and 0.020 mol H2 are placed in a 3.50-L vessel at 1260 K.What is the equilibrium partial pressure of CO(g)? (R = 0.0821 L ∙ atm/(K ∙ mol))
A)4.1 atm
B)0.5 atm
C)1.6 atm
D)1.1 atm
E)0.59 atm

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
74
Consider the following equilibrium:
PCl5(g)
PCl3(g)+ Cl2 (g); ΔH = 92 kJ
The concentration of PCl3 at equilibrium may be increased by
A)decreasing the temperature.
B)adding Cl2 to the system.
C)adding PCl5 to the system.
D)increasing the pressure.
E)adding a catalyst.
PCl5(g)

PCl3(g)+ Cl2 (g); ΔH = 92 kJ
The concentration of PCl3 at equilibrium may be increased by
A)decreasing the temperature.
B)adding Cl2 to the system.
C)adding PCl5 to the system.
D)increasing the pressure.
E)adding a catalyst.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
75
When the pressure of an equilibrium mixture of SO2,O2,and SO3 is halved at constant temperature,what is the effect on Kp?
2SO2(g)+ O2(g)
2SO3(g)
A)Kp is doubled.
B)Kp is halved.
C)Kp is unchanged.
D)Kp is tripled.
E)Kp is decreased by a third.
2SO2(g)+ O2(g)

2SO3(g)
A)Kp is doubled.
B)Kp is halved.
C)Kp is unchanged.
D)Kp is tripled.
E)Kp is decreased by a third.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
76
Exactly 1.0 mol N2O4 is placed in an empty 1.0-L container and allowed to reach equilibrium described by the equation N2O4(g)  2NO2(g).
2NO2(g).
If at equilibrium the N2O4 is 28.0% dissociated,what is the value of the equilibrium constant,Kc,for the reaction under these conditions?
A)0.44
B)2.3
C)0.31
D)0.78
E)0.11
 2NO2(g).
2NO2(g).If at equilibrium the N2O4 is 28.0% dissociated,what is the value of the equilibrium constant,Kc,for the reaction under these conditions?
A)0.44
B)2.3
C)0.31
D)0.78
E)0.11

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
77
Carbon monoxide is toxic because it can successfully compete with oxygen for hemoglobin (Hb)sites according to the following equilibrium:
Hb(O2)4(aq)+ 4CO(g)
Hb(CO)4(aq)+ 4O2(g)
From Le Châtelier's principle,CO poisoning is reversed by
A)increasing the O2 pressure.
B)decreasing the amount of Hb.
C)increasing the CO2 pressure.
D)increasing the CO pressure.
E)increasing the amount of Hb.
Hb(O2)4(aq)+ 4CO(g)

Hb(CO)4(aq)+ 4O2(g)
From Le Châtelier's principle,CO poisoning is reversed by
A)increasing the O2 pressure.
B)decreasing the amount of Hb.
C)increasing the CO2 pressure.
D)increasing the CO pressure.
E)increasing the amount of Hb.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
78
For a specific reaction,which of the following statements can be made about the equilibrium constant?
A)It can be changed by the addition of a catalyst.
B)It increases when the concentration of one of the products is increased.
C)It increases when the concentration of one of the reactants is increased.
D)It always remains the same.
E)It changes with changes in the temperature.
A)It can be changed by the addition of a catalyst.
B)It increases when the concentration of one of the products is increased.
C)It increases when the concentration of one of the reactants is increased.
D)It always remains the same.
E)It changes with changes in the temperature.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
79
When cobalt chloride is added to pure water,the Co2+ ions hydrate.The hydrated form then reacts with the Cl- ions to set up the equilibrium shown here:
Co(H2O)62+ + 4Cl-
CoCl42- + 6H2O
(pink)
(blue)
Which statement describes the change that the system will undergo if potassium chloride is added?
A)It should become more pink.
B)Nothing will change.
C)The silver ion will react with the CoCl42-.
D)Water will be produced.
E)It should become more blue.
Co(H2O)62+ + 4Cl-

CoCl42- + 6H2O
(pink)
(blue)
Which statement describes the change that the system will undergo if potassium chloride is added?
A)It should become more pink.
B)Nothing will change.
C)The silver ion will react with the CoCl42-.
D)Water will be produced.
E)It should become more blue.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
80
In which of the following reactions does a decrease in the volume of the reaction vessel at constant temperature favor formation of the products?
A)2H2(g)+ O2(g) 2H2O(g)
2H2O(g)
B)NO2(g)+ CO(g) NO(g)+ CO2(g)
NO(g)+ CO2(g)
C)H2(g)+ I2(g) 2HI(g)
2HI(g)
D)2O3(g) 3O2(g)
3O2(g)
E)MgCO3(s) MgO(s)+ CO2(g)
MgO(s)+ CO2(g)
A)2H2(g)+ O2(g)
 2H2O(g)
2H2O(g)B)NO2(g)+ CO(g)
 NO(g)+ CO2(g)
NO(g)+ CO2(g)C)H2(g)+ I2(g)
 2HI(g)
2HI(g)D)2O3(g)
 3O2(g)
3O2(g)E)MgCO3(s)
 MgO(s)+ CO2(g)
MgO(s)+ CO2(g)
Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck


