Exam 14: Chemical Equilibrium
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
For the equilibrium N2O4(g)  2NO2(g),at 298 K,Kp = 0.15.For this reaction system,it is found that the partial pressure of N2O4 is 3.5 × 10-2 atm at equilibrium.What is the partial pressure of NO2 at equilibrium? (R = 0.0821 L ∙ atm/(K ∙ mol))
2NO2(g),at 298 K,Kp = 0.15.For this reaction system,it is found that the partial pressure of N2O4 is 3.5 × 10-2 atm at equilibrium.What is the partial pressure of NO2 at equilibrium? (R = 0.0821 L ∙ atm/(K ∙ mol))
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
E
What balanced equation is the following equilibrium expression derived from? 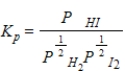
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
A
For which of the following systems at equilibrium and at constant temperature will decreasing the volume cause the equilibrium to shift to the right?
Free
(Multiple Choice)
4.7/5  (33)
(33)
Correct Answer:
D
Consider the following equilibrium:
2NO(g)+ 3F2(g)  2NOF3(g)
Suppose 0.20 mol of NO and 0.30 mol of F2 are added to a 5.0-L container.If x mol of NOF3 is present at equilibrium,how many moles of fluorine are present at equilibrium?
2NOF3(g)
Suppose 0.20 mol of NO and 0.30 mol of F2 are added to a 5.0-L container.If x mol of NOF3 is present at equilibrium,how many moles of fluorine are present at equilibrium?
(Multiple Choice)
4.9/5  (31)
(31)
Which of the following equilibria would not be affected by pressure changes at constant temperature?
(Multiple Choice)
4.8/5  (29)
(29)
What is the expression for Kc for the following equilibrium?
CaSO3(s)  CaO(s)+ SO2(g)
CaO(s)+ SO2(g)
(Multiple Choice)
4.8/5  (33)
(33)
One method for the decomposition of carbon dioxide proceeds as follows:
2CO2(g)  2CO(g)+ O2(g); ΔH = 559 kJ
Which of the following changes will cause an increase in the equilibrium concentration of CO?
2CO(g)+ O2(g); ΔH = 559 kJ
Which of the following changes will cause an increase in the equilibrium concentration of CO?
(Multiple Choice)
4.9/5  (39)
(39)
Exactly 1.0 mol N2O4 is placed in an empty 1.0-L container and allowed to reach equilibrium described by the equation N2O4(g)  2NO2(g).
If at equilibrium the N2O4 is 28.0% dissociated,what is the value of the equilibrium constant,Kc,for the reaction under these conditions?
2NO2(g).
If at equilibrium the N2O4 is 28.0% dissociated,what is the value of the equilibrium constant,Kc,for the reaction under these conditions?
(Multiple Choice)
5.0/5  (33)
(33)
Which of the following equilibria would be affected by volume changes at constant temperature?
(Multiple Choice)
4.9/5  (33)
(33)
Solid HgO,liquid Hg,and gaseous O2 are placed in a glass bulb and allowed to reach equilibrium.
2HgO(s)  2Hg(l)+ O2(g); ΔH = 181.6 kJ
The amount of Hg(l)in the bulb could be increased
2Hg(l)+ O2(g); ΔH = 181.6 kJ
The amount of Hg(l)in the bulb could be increased
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following statements is true in a reaction system at equilibrium?
(Multiple Choice)
4.9/5  (30)
(30)
What is the balanced equation for the following equilibrium expression? 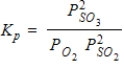
(Multiple Choice)
4.8/5  (31)
(31)
For which of the following values of the equilibrium constant does the reaction mixture contain mostly products?
(Multiple Choice)
4.9/5  (34)
(34)
Consider the following system at equilibrium: N2(g)+ 3H2(g)  2NH3(g); ΔH° = -92.94 kJ Which of the following changes will shift the equilibrium to the right?
I.increasing the temperature
II.decreasing the temperature
III.increasing the volume
IV.decreasing the volume
V.removing some NH3
VI.adding some NH3
VII.removing some N2
VIII.adding some N2
2NH3(g); ΔH° = -92.94 kJ Which of the following changes will shift the equilibrium to the right?
I.increasing the temperature
II.decreasing the temperature
III.increasing the volume
IV.decreasing the volume
V.removing some NH3
VI.adding some NH3
VII.removing some N2
VIII.adding some N2
(Multiple Choice)
4.8/5  (36)
(36)
The reaction quotient for a system is 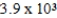 .If the equilibrium constant for the system is
.If the equilibrium constant for the system is 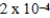 ,what will happen as the reaction mixture approaches equilibrium?
,what will happen as the reaction mixture approaches equilibrium?
(Multiple Choice)
4.7/5  (40)
(40)
Consider the reaction
S2Cl2(l)+ CCl4(l)  CS2(g)+ 3Cl2(g); ΔH° = 84.3 kJ
If the above reactants and products are contained in a closed vessel and the reaction system is at equilibrium,the number of moles of CS2 can be increased by
CS2(g)+ 3Cl2(g); ΔH° = 84.3 kJ
If the above reactants and products are contained in a closed vessel and the reaction system is at equilibrium,the number of moles of CS2 can be increased by
(Multiple Choice)
4.9/5  (41)
(41)
Which expression correctly describes the equilibrium constant Kc for the following reaction?
2C2H2(g)+ 5O2(g)  4CO2(g)+ 2H2O(g)
4CO2(g)+ 2H2O(g)
(Multiple Choice)
4.9/5  (36)
(36)
Consider the following equilibrium:
1/2N2O4(g)  NO2(g); Kc = 3.3 at 100°C
For which of the following equilibria is Kc less than 3.3 at 100°C?
NO2(g); Kc = 3.3 at 100°C
For which of the following equilibria is Kc less than 3.3 at 100°C?
(Multiple Choice)
4.9/5  (38)
(38)
At 298 K,the value of Kc for the reaction H2(g)+ Br2(g)  2HBr(g)is 2.0 × 1019.What is Kc for HBr(g)
2HBr(g)is 2.0 × 1019.What is Kc for HBr(g)  1/2H2(g)+ 1/2Br2(g)?
1/2H2(g)+ 1/2Br2(g)?
(Multiple Choice)
4.9/5  (30)
(30)
A(n)_____ is a substance that increases the rate of a reaction but is not consumed by it.
(Multiple Choice)
4.9/5  (46)
(46)
Showing 1 - 20 of 97
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)