Deck 16: Acid-Base Equilibria
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/148
Play
Full screen (f)
Deck 16: Acid-Base Equilibria
1
A 8.0 × 10-3 M solution of acetic acid,HC2H3O2,is 4.7% ionized at 25°C.In a 8.0 × 10-4 M solution,the percentage of ionization would be
A)100%.
B)<4.7%.
C)>4.7%.
D)the same.
E)zero.
A)100%.
B)<4.7%.
C)>4.7%.
D)the same.
E)zero.
>4.7%.
2
For which of the following equilibria does Kc correspond to the acid-ionization constant,Ka,of HC2O4-?
A)HC2O4-(aq)+ H2O(l) H2C2O4(aq)+ OH-(aq)
H2C2O4(aq)+ OH-(aq)
B)H2C2O4(aq)+ H2O(l) H3O+(aq)+ HC2O4-(aq)
H3O+(aq)+ HC2O4-(aq)
C)HC2O4-(aq)+ H2O(l) H3O+(aq)+ C2O42-(aq)
H3O+(aq)+ C2O42-(aq)
D)HC2O4-(aq)+ OH-(aq) C2O42-(aq)+ H2O(l)
C2O42-(aq)+ H2O(l)
E)HC2O4-(aq)+ H3O+(aq) H2C2O4(aq)+ H2O(l)
H2C2O4(aq)+ H2O(l)
A)HC2O4-(aq)+ H2O(l)
 H2C2O4(aq)+ OH-(aq)
H2C2O4(aq)+ OH-(aq)B)H2C2O4(aq)+ H2O(l)
 H3O+(aq)+ HC2O4-(aq)
H3O+(aq)+ HC2O4-(aq)C)HC2O4-(aq)+ H2O(l)
 H3O+(aq)+ C2O42-(aq)
H3O+(aq)+ C2O42-(aq)D)HC2O4-(aq)+ OH-(aq)
 C2O42-(aq)+ H2O(l)
C2O42-(aq)+ H2O(l)E)HC2O4-(aq)+ H3O+(aq)
 H2C2O4(aq)+ H2O(l)
H2C2O4(aq)+ H2O(l)HC2O4-(aq)+ H2O(l)  H3O+(aq)+ C2O42-(aq)
H3O+(aq)+ C2O42-(aq)
 H3O+(aq)+ C2O42-(aq)
H3O+(aq)+ C2O42-(aq) 3
Consider the Ka values for the following acids:
Cyanic acid,HOCN,3.5 × 10-4
Formic acid,HCHO2,1.7 × 10-4
Lactic acid,HC3H5O3,1.3 × 10-4
Propionic acid,HC3H5O2,1.3 × 10-5
Benzoic acid,HC7H5O2,6.3 × 10-5
Which has the strongest conjugate base?
A)propionic acid
B)benzoic acid
C)lactic acid
D)formic acid
E)cyanic
Cyanic acid,HOCN,3.5 × 10-4
Formic acid,HCHO2,1.7 × 10-4
Lactic acid,HC3H5O3,1.3 × 10-4
Propionic acid,HC3H5O2,1.3 × 10-5
Benzoic acid,HC7H5O2,6.3 × 10-5
Which has the strongest conjugate base?
A)propionic acid
B)benzoic acid
C)lactic acid
D)formic acid
E)cyanic
propionic acid
4
Consider the Ka values for the following acids:
Cyanic acid,HOCN,3.5 × 10-4
Formic acid,HCHO2,1.7 × 10-4
Lactic acid,HC3H5O3,1.3 × 10-4
Propionic acid,HC3H5O2,1.3 × 10-5
Benzoic acid,HC7H5O2,6.3 × 10-5
Given initially equimolar soutions of each weak acid,which solution will have the highest hydronium ion concentration once equilibrium is established?
A)cyanic acid
B)benzoic acid
C)lactic acid
D)formic acid
E)propionic acid
Cyanic acid,HOCN,3.5 × 10-4
Formic acid,HCHO2,1.7 × 10-4
Lactic acid,HC3H5O3,1.3 × 10-4
Propionic acid,HC3H5O2,1.3 × 10-5
Benzoic acid,HC7H5O2,6.3 × 10-5
Given initially equimolar soutions of each weak acid,which solution will have the highest hydronium ion concentration once equilibrium is established?
A)cyanic acid
B)benzoic acid
C)lactic acid
D)formic acid
E)propionic acid

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
5
A 0.10 M aqueous solution of a weak acid HA has a pH of 5.00.What is the value of Ka for HA?
A)1.0 × 10-8
B)1.0 × 10-6
C)1.0 × 10-7
D)1.0 × 10-5
E)1.0 × 10-9
A)1.0 × 10-8
B)1.0 × 10-6
C)1.0 × 10-7
D)1.0 × 10-5
E)1.0 × 10-9

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
6
For which of the following equilibria does Kc correspond to an acid-ionization constant,Ka?
A)NH3(aq)+ H3O+(aq) NH4+(aq)+ H2O(l)
NH4+(aq)+ H2O(l)
B)NH4+(aq)+ OH-(aq) NH3(aq)+ H2O(l)
NH3(aq)+ H2O(l)
C)F-(aq)+ H2O(l) HF(aq)+ OH-(aq)
HF(aq)+ OH-(aq)
D)HF(aq)+ OH-(aq) H2O(l)+ F-(aq)
H2O(l)+ F-(aq)
E)NH4+(aq)+ H2O(l) NH3(aq)+ H3O+(aq)
NH3(aq)+ H3O+(aq)
A)NH3(aq)+ H3O+(aq)
 NH4+(aq)+ H2O(l)
NH4+(aq)+ H2O(l)B)NH4+(aq)+ OH-(aq)
 NH3(aq)+ H2O(l)
NH3(aq)+ H2O(l)C)F-(aq)+ H2O(l)
 HF(aq)+ OH-(aq)
HF(aq)+ OH-(aq)D)HF(aq)+ OH-(aq)
 H2O(l)+ F-(aq)
H2O(l)+ F-(aq)E)NH4+(aq)+ H2O(l)
 NH3(aq)+ H3O+(aq)
NH3(aq)+ H3O+(aq)
Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
7
For the equilibrium that exists in an aqueous solution of nitrous acid (HNO2,a weak acid),the equilibrium-constant expression is
A)K =![<strong>For the equilibrium that exists in an aqueous solution of nitrous acid (HNO<sub>2</sub>,a weak acid),the equilibrium-constant expression is</strong> A)K = . B)K = . C)K = . D)K = [H<sup>+</sup>][NO<sub>2</sub><sup>-</sup>]. E)none of these](https://storage.examlex.com/TB2288/11ea7a3a_9f23_da3e_a82d_750e6e2906ff_TB2288_11.jpg) .
.
B)K =![<strong>For the equilibrium that exists in an aqueous solution of nitrous acid (HNO<sub>2</sub>,a weak acid),the equilibrium-constant expression is</strong> A)K = . B)K = . C)K = . D)K = [H<sup>+</sup>][NO<sub>2</sub><sup>-</sup>]. E)none of these](https://storage.examlex.com/TB2288/11ea7a3a_9f24_014f_a82d_0de25bbd5001_TB2288_11.jpg) .
.
C)K =![<strong>For the equilibrium that exists in an aqueous solution of nitrous acid (HNO<sub>2</sub>,a weak acid),the equilibrium-constant expression is</strong> A)K = . B)K = . C)K = . D)K = [H<sup>+</sup>][NO<sub>2</sub><sup>-</sup>]. E)none of these](https://storage.examlex.com/TB2288/11ea7a3a_9f24_0150_a82d_49d3790b09aa_TB2288_11.jpg) .
.
D)K = [H+][NO2-].
E)none of these
A)K =
![<strong>For the equilibrium that exists in an aqueous solution of nitrous acid (HNO<sub>2</sub>,a weak acid),the equilibrium-constant expression is</strong> A)K = . B)K = . C)K = . D)K = [H<sup>+</sup>][NO<sub>2</sub><sup>-</sup>]. E)none of these](https://storage.examlex.com/TB2288/11ea7a3a_9f23_da3e_a82d_750e6e2906ff_TB2288_11.jpg) .
.B)K =
![<strong>For the equilibrium that exists in an aqueous solution of nitrous acid (HNO<sub>2</sub>,a weak acid),the equilibrium-constant expression is</strong> A)K = . B)K = . C)K = . D)K = [H<sup>+</sup>][NO<sub>2</sub><sup>-</sup>]. E)none of these](https://storage.examlex.com/TB2288/11ea7a3a_9f24_014f_a82d_0de25bbd5001_TB2288_11.jpg) .
.C)K =
![<strong>For the equilibrium that exists in an aqueous solution of nitrous acid (HNO<sub>2</sub>,a weak acid),the equilibrium-constant expression is</strong> A)K = . B)K = . C)K = . D)K = [H<sup>+</sup>][NO<sub>2</sub><sup>-</sup>]. E)none of these](https://storage.examlex.com/TB2288/11ea7a3a_9f24_0150_a82d_49d3790b09aa_TB2288_11.jpg) .
.D)K = [H+][NO2-].
E)none of these

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
8
For which of the following equilibria does Kc correspond to the acid-dissociation constant,Ka,of H2PO4-?
A)H2PO4-(aq)+ H3O+(aq) H3PO4(aq)+ H2O(l)
H3PO4(aq)+ H2O(l)
B)H2PO4-(aq)+ H2O(l) H3PO4(aq)+ OH-(aq)
H3PO4(aq)+ OH-(aq)
C)H2PO4-(aq)+ H2O(l) H3O+(aq)+ HPO42-(aq)
H3O+(aq)+ HPO42-(aq)
D)H3PO4(aq)+ H2O(l) H3O+(aq)+ H2PO4-(aq)
H3O+(aq)+ H2PO4-(aq)
E)HPO42-(aq)+ H2O(l) H2PO4-(aq)+ OH-(aq)
H2PO4-(aq)+ OH-(aq)
A)H2PO4-(aq)+ H3O+(aq)
 H3PO4(aq)+ H2O(l)
H3PO4(aq)+ H2O(l)B)H2PO4-(aq)+ H2O(l)
 H3PO4(aq)+ OH-(aq)
H3PO4(aq)+ OH-(aq)C)H2PO4-(aq)+ H2O(l)
 H3O+(aq)+ HPO42-(aq)
H3O+(aq)+ HPO42-(aq)D)H3PO4(aq)+ H2O(l)
 H3O+(aq)+ H2PO4-(aq)
H3O+(aq)+ H2PO4-(aq)E)HPO42-(aq)+ H2O(l)
 H2PO4-(aq)+ OH-(aq)
H2PO4-(aq)+ OH-(aq)
Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
9
Rank acetic acid (HC2H3O2),hydrocyanic acid (HOCN),and hydrofluoric acid (HF)in order of increasing strength.
Acid
Ka
HC2H3O2
1)8 × 10-5
HOCN
3)5 × 10-4
HF
6)8 × 10-4
A)HC2H3O2 < HOCN < HF
B)HOCN < HC2H3O2 < HF
C)HOCN < HF < HC2H3O2
D)HF < HOCN < HC2H3O2
E)HF < HC2H3O2 < HOCN
Acid
Ka
HC2H3O2
1)8 × 10-5
HOCN
3)5 × 10-4
HF
6)8 × 10-4
A)HC2H3O2 < HOCN < HF
B)HOCN < HC2H3O2 < HF
C)HOCN < HF < HC2H3O2
D)HF < HOCN < HC2H3O2
E)HF < HC2H3O2 < HOCN

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
10
What is the equilibrium expression for the equilibrium A-(aq)+ H3O+(aq)  HA(aq)+ H2O(l)?
HA(aq)+ H2O(l)?
A)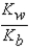
B)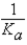
C)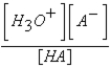
D)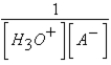
E)Kb
 HA(aq)+ H2O(l)?
HA(aq)+ H2O(l)?A)
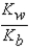
B)
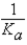
C)
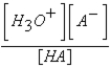
D)
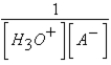
E)Kb

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
11
What is the percent ionization of a 1.5 M HC2H3O2 solution (Ka = 1.8 × 10-5 )at 25°C?
+
A)0.52 %
B)0.35 %
C)2.5 %
D)0.18 %
E)0.28 %
+
A)0.52 %
B)0.35 %
C)2.5 %
D)0.18 %
E)0.28 %

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
12
A 0.010 M aqueous solution of a weak acid HA has a pH of 4.0.What is the degree of ionization of HA in the solution?
A)0.1 %
B)0.01 %
C)0.001 %
D)1 %
E)10 %
A)0.1 %
B)0.01 %
C)0.001 %
D)1 %
E)10 %

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
13
For which of the following equilibria does Kc correspond to the acid-ionization constant,Ka,of HCO3-?
A)HCO3-(aq)+ OH-(aq) CO32-(aq)+ H2O(l)
CO32-(aq)+ H2O(l)
B)H2CO3(aq)+ H2O(l) HCO3-(aq)+ H3O+(aq)
HCO3-(aq)+ H3O+(aq)
C)HCO3-(aq)+ H2O(l) H2CO3(aq)+ OH-(aq)
H2CO3(aq)+ OH-(aq)
D)HCO3-(aq)+ H3O+(aq) H2CO3(aq)+ H2O(l)
H2CO3(aq)+ H2O(l)
E)HCO3-(aq)+ H2O(l) CO32-(aq)+ H3O+(aq)
CO32-(aq)+ H3O+(aq)
A)HCO3-(aq)+ OH-(aq)
 CO32-(aq)+ H2O(l)
CO32-(aq)+ H2O(l)B)H2CO3(aq)+ H2O(l)
 HCO3-(aq)+ H3O+(aq)
HCO3-(aq)+ H3O+(aq)C)HCO3-(aq)+ H2O(l)
 H2CO3(aq)+ OH-(aq)
H2CO3(aq)+ OH-(aq)D)HCO3-(aq)+ H3O+(aq)
 H2CO3(aq)+ H2O(l)
H2CO3(aq)+ H2O(l)E)HCO3-(aq)+ H2O(l)
 CO32-(aq)+ H3O+(aq)
CO32-(aq)+ H3O+(aq)
Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
14
A 0.10 M solution of a weak monoprotic acid has a hydronium-ion concentration of 7.2 × 10-4 M.What is the acid-ionization constant,Ka,for this acid?
A)2.7 × 10-2
B)6.3 × 10-3
C)7.2 × 10-4
D)5.2 × 10-6
E)8.6 × 10-5
A)2.7 × 10-2
B)6.3 × 10-3
C)7.2 × 10-4
D)5.2 × 10-6
E)8.6 × 10-5

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
15
An initially 1.0 M aqueous solution of a weak monoprotic acid has a total ion concentration of 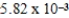 M when equilibrium is established.What is the acid-ionization constant,Ka,of the weak acid? (assume Ca/Ka ≥ 102)
M when equilibrium is established.What is the acid-ionization constant,Ka,of the weak acid? (assume Ca/Ka ≥ 102)
A)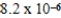
B)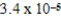
C)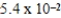
D)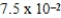
E)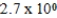
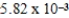 M when equilibrium is established.What is the acid-ionization constant,Ka,of the weak acid? (assume Ca/Ka ≥ 102)
M when equilibrium is established.What is the acid-ionization constant,Ka,of the weak acid? (assume Ca/Ka ≥ 102)A)
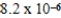
B)
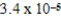
C)
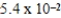
D)
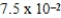
E)
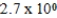

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
16
At a temperature of 25°C an initally 0.011 M solution of a weak monoprotic acid is 2.9 % ionized once equilibrium is established.What is the acid-ionization constant,Ka,for this acid? (assume Ca/Ka ≥ 102)
A)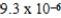
B)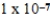
C)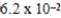
D)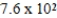
E)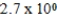
A)
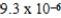
B)
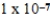
C)
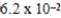
D)
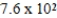
E)
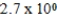

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
17
What is the pH of an initially 0.428 M solution of a weak monoprotic acid that is 0.11 % ionized when equilibrium is established? (assume Ca/Ka ≥102)
A)3.32
B)7.00
C)1.32
D)10.63
E)10.68
A)3.32
B)7.00
C)1.32
D)10.63
E)10.68

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
18
Equal moles of the indicated acids are dissolved in the amounts of water shown in the beakers below.In which solution will the percent ionization of the acid be the lowest? 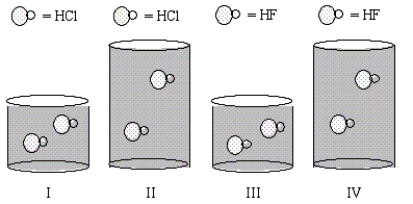
A)All have equal percent ionization of acid.
B)III
C)IV
D)II
E)I

A)All have equal percent ionization of acid.
B)III
C)IV
D)II
E)I

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
19
A 0.10 M solution of a weak monoprotic acid has a pH of 3.40 at 25°C.What is the acid-ionization constant,Ka,for this acid?
A)1.6 × 10-6
B)4.0 × 10-4
C)3.4 × 10-5
D)1.2 × 10-3
E)1.8 × 10-7
A)1.6 × 10-6
B)4.0 × 10-4
C)3.4 × 10-5
D)1.2 × 10-3
E)1.8 × 10-7

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
20
A 0.20 M solution of a weak monoprotic acid is 0.22 % ionized.What is the acid-ionization constant,Ka,for this acid?
A)1.9 × 10-6
B)2.2 × 10-6
C)1.1 × 10-4
D)2.4 × 10-5
E)9.7 × 10-7
A)1.9 × 10-6
B)2.2 × 10-6
C)1.1 × 10-4
D)2.4 × 10-5
E)9.7 × 10-7

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
21
What is the equilibrium hydronium ion concentration of an initially 4.5 M solution of hypoiodous acid,HOI,at 25°C (Ka = 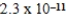 )?
)?
A)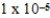 M
M
B)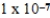 M
M
C)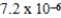 M
M
D)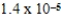 M
M
E)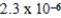 M
M
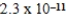 )?
)?A)
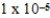 M
MB)
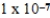 M
MC)
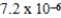 M
MD)
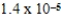 M
ME)
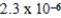 M
M
Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
22
What is the equilibrium pH of an initially 0.73 M solution of the monoprotic acid benzoic acid at 25°C (Ka = 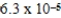 )?
)?
A)2.17
B)7.00
C)1.87
D)12.13
E)5.13
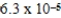 )?
)?A)2.17
B)7.00
C)1.87
D)12.13
E)5.13

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
23
What is the equilibrium percent ionization of an initially 0.32 M solution of the monoprotic acid valeric acid,HC5H9O2,at 25°C (Ka = 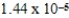 )?
)?
A)0.67%
B)1.30%
C)0.0014%
D)0.1%
E)2.00%
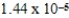 )?
)?A)0.67%
B)1.30%
C)0.0014%
D)0.1%
E)2.00%

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
24
In a 0.20 M solution of a diprotic acid H2A (Ka1 = 1.3 × 10-5 and Ka2 = 2.7 × 10-10 at 25°C),what is the equilibrium concentration of A2-?
A)7.3 × 10-6 M
B)2.7 × 10-10 M
C)1.6 × 10-3 M
D)0.40 M
E)0.20 M
A)7.3 × 10-6 M
B)2.7 × 10-10 M
C)1.6 × 10-3 M
D)0.40 M
E)0.20 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
25
What is Ka for a weak monoprotic acid if a 0.020 M solution of the acid has a pH of 3.28 at 25°C?
A)5.2 × 10-2
B)1.4 × 10-5
C)7.1 × 10-2
D)1.0 × 10-6
E)2.8 × 10-4
A)5.2 × 10-2
B)1.4 × 10-5
C)7.1 × 10-2
D)1.0 × 10-6
E)2.8 × 10-4

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
26
In a 0.01 M solution of 1,4-butanedicarboxylic acid,HOOCCH2CH2COOH (Ka1 = 2.9 × 10-5,Ka2 = 5.3 × 10-6),which species is present in the lowest concentration?
A)(-OOCCH2CH2COO-)(aq)
B)H2O
C)HOOCCH2CH2COO-(aq)
D)H3O+(aq)
E)HOOCCH2CH2COOH(aq)
A)(-OOCCH2CH2COO-)(aq)
B)H2O
C)HOOCCH2CH2COO-(aq)
D)H3O+(aq)
E)HOOCCH2CH2COOH(aq)

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
27
What is the pH of a solution prepared by dissolving 0.0163 mol of chloroacetic acid,(HC2H2O2Cl)in 1.30 L of water? For chloroacetic acid,Ka = 1.4 × 10-3.
A)1.90
B)2.45
C)11.55
D)12.10
E)2.38
A)1.90
B)2.45
C)11.55
D)12.10
E)2.38

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
28
In a 0.01 M solution of 1,4-butanedicarboxylic acid,HOOCCH2CH2COOH (Ka1 = 2.9 × 10-5,Ka2 = 5.3 × 10-6),which species is present in the highest concentration?
A)HOOCCH2CH2COO-(aq)
B)HOOCCH2CH2COOH(aq)
C)H3O+(aq)
D)(-OOCCH2CH2COO-)(aq)
E)OH-(aq)
A)HOOCCH2CH2COO-(aq)
B)HOOCCH2CH2COOH(aq)
C)H3O+(aq)
D)(-OOCCH2CH2COO-)(aq)
E)OH-(aq)

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
29
What is the equilibrium pH of an initially 4.4 M solution of hypochlorous acid,HOCl,at 25°C (Ka = 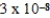 )?
)?
A)3.44
B)7.37
C)10.41
D)10.71
E)4.08
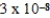 )?
)?A)3.44
B)7.37
C)10.41
D)10.71
E)4.08

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
30
Carbonic acid,H2CO3,is a weak diprotic acid.In a 0.1 M solution of the acid,which of the following species is present in the largest amount?
A)HCO3-
B)CO32-
C)H2CO3
D)H3O+
E)OH-
A)HCO3-
B)CO32-
C)H2CO3
D)H3O+
E)OH-

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
31
Phosphoric acid,H3PO4,will undergo three successive ionization reactions to varying extents in water.What is the balanced equilibrium identified as Ka3?
A)H2PO4-(aq)+ H2O(l) H3O+(aq)+ HPO42-(aq)
H3O+(aq)+ HPO42-(aq)
B)HPO42-(aq)+ H2O(l) PO43-(aq)+ H3O+(aq)
PO43-(aq)+ H3O+(aq)
C)H2PO4-(aq)+ H3O+(aq) H3PO4(aq)+ H2O(l)
H3PO4(aq)+ H2O(l)
D)H3PO4(aq)+ H2O(l) H3O+(aq)+ H2PO4-(aq)
H3O+(aq)+ H2PO4-(aq)
E)PO43-(aq)+ H2O(l) HPO42-(aq)+ OH-(aq)
HPO42-(aq)+ OH-(aq)
A)H2PO4-(aq)+ H2O(l)
 H3O+(aq)+ HPO42-(aq)
H3O+(aq)+ HPO42-(aq)B)HPO42-(aq)+ H2O(l)
 PO43-(aq)+ H3O+(aq)
PO43-(aq)+ H3O+(aq)C)H2PO4-(aq)+ H3O+(aq)
 H3PO4(aq)+ H2O(l)
H3PO4(aq)+ H2O(l)D)H3PO4(aq)+ H2O(l)
 H3O+(aq)+ H2PO4-(aq)
H3O+(aq)+ H2PO4-(aq)E)PO43-(aq)+ H2O(l)
 HPO42-(aq)+ OH-(aq)
HPO42-(aq)+ OH-(aq)
Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
32
For a 0.05 M H2SO3 solution,which of the following relationships is true?
A)[SO32-] > [H2SO3]
B)[SO32-] > [HSO3-]
C)[HSO3-] > [H2SO3]
D)[H2SO3] > [H+]
E)[H+] > [H2SO3]
A)[SO32-] > [H2SO3]
B)[SO32-] > [HSO3-]
C)[HSO3-] > [H2SO3]
D)[H2SO3] > [H+]
E)[H+] > [H2SO3]

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
33
A 8.42-g sample of homogentisic acid,a weak organic acid having Ka = 4.0 × 10-5,is dissolved in 35.00 mL of water and its pH is measured to be 2.122.What is the molar mass of homogentisic acid?
A)168 g/mol
B)1110 g/mol
C)1.43 g/mol
D)5.88 g/mol
E)240 g/mol
A)168 g/mol
B)1110 g/mol
C)1.43 g/mol
D)5.88 g/mol
E)240 g/mol

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
34
What is the equilibrium concentration of chloroacetic acid,HC2H2O2Cl,in a solution prepared by dissolving 0.0271 mol of HC2H2O2Cl in 1.50 L of water? For chloroacetic acid,Ka = 1.4 × 10-3.
A)4.38 × 10-3 M
B)1.81 × 10-2 M
C)5.03 × 10-3 M
D)1.37 × 10-2 M
E)9.03 × 10-3 M
A)4.38 × 10-3 M
B)1.81 × 10-2 M
C)5.03 × 10-3 M
D)1.37 × 10-2 M
E)9.03 × 10-3 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following represents the usual relationship of acid-ionization constants for a triprotic acid?
A)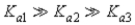
B)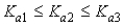
C)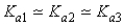
D)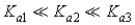
E)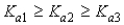
A)
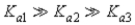
B)
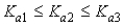
C)
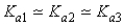
D)
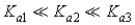
E)
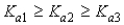

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
36
It is safe to make the simplifying assumption that x can be neglected in the denominator of the equilibrium equation when
A)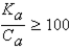 .
.
B)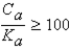 .
.
C)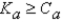 .
.
D)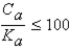 .
.
E)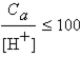 .
.
A)
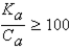 .
.B)
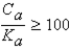 .
.C)
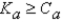 .
.D)
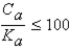 .
.E)
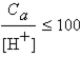 .
.
Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
37
What is the percent ionization of a solution prepared by dissolving 0.0175 mol of chloroacetic acid,(HC2H2O2Cl)in 1.30 L of water? For chloroacetic acid,Ka = 1.4 × 10-3.
A)1.3 %
B)100 %
C)27 %
D)72 %
E)0.36 %
A)1.3 %
B)100 %
C)27 %
D)72 %
E)0.36 %

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
38
For a 0.10 M solution of glutaric acid,HO2C(CH2)3CO2H (Ka1 = 4.6 × 10-5,Ka2 = 3.9 × 10-6),rank the following species in order of increasing equilibrium concentration.
A)H3O+ < -O2C(CH2)3COO- < HO2C(CH2)3COO- < OH- < HO2C(CH2)3CO2H
B)OH- < -O2C(CH2)3COO- < H3O+ < HO2C(CH2)3COO- < HO2C(CH2)3CO2H
C)OH- < -O2C(CH2)3COO- < HO2C(CH2)3COO- < H3O+ < HO2C(CH2)3CO2H
D)H3O+ < HO2C(CH2)3CO2H < HO2C(CH2)3COO- < -O2C(CH2)3COO- < OH-
E)OH- < -O2C(CH2)3COO- < HO2C(CH2)3COO- < HO2C(CH2)3CO2H < H3O+
A)H3O+ < -O2C(CH2)3COO- < HO2C(CH2)3COO- < OH- < HO2C(CH2)3CO2H
B)OH- < -O2C(CH2)3COO- < H3O+ < HO2C(CH2)3COO- < HO2C(CH2)3CO2H
C)OH- < -O2C(CH2)3COO- < HO2C(CH2)3COO- < H3O+ < HO2C(CH2)3CO2H
D)H3O+ < HO2C(CH2)3CO2H < HO2C(CH2)3COO- < -O2C(CH2)3COO- < OH-
E)OH- < -O2C(CH2)3COO- < HO2C(CH2)3COO- < HO2C(CH2)3CO2H < H3O+

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
39
What is the hydronium-ion concentration of a 0.30 M solution of HCN (Ka = 4.9 × 10-10)at 25°C?
A)1.7 × 10-4 M
B)3.4 × 10-6 M
C)2.2 × 10-6 M
D)1.2 × 10-5 M
E)4.0 × 10-5 M
A)1.7 × 10-4 M
B)3.4 × 10-6 M
C)2.2 × 10-6 M
D)1.2 × 10-5 M
E)4.0 × 10-5 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
40
The equilibrium hydronium ion concentration of an initially 0.655 M solution of a monoprotic weak acid is 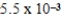 M.The acid dissociation constant is
M.The acid dissociation constant is 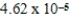
At 25°C.What is the pH of this solution?
A)2.26
B)7.00
C)11.74
D)4.52
E)0.18
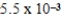 M.The acid dissociation constant is
M.The acid dissociation constant is 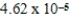
At 25°C.What is the pH of this solution?
A)2.26
B)7.00
C)11.74
D)4.52
E)0.18

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
41
What is the hydronium-ion concentration of a 0.210 M oxalic acid,H2C2O4,solution? For oxalic acid,Ka1 = 5.6 × 10-2 and Ka2 = 5.1 × 10-5.
A)8.4 × 10-2 M
B)1.1 × 10-1 M
C)1.0 × 10-7 M
D)3.2 × 10-3 M
E)3.3 × 10-3 M
A)8.4 × 10-2 M
B)1.1 × 10-1 M
C)1.0 × 10-7 M
D)3.2 × 10-3 M
E)3.3 × 10-3 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following reactions is associated with the definition of Kb?
A)CN-(aq)+ H+(aq)![<strong>Which of the following reactions is associated with the definition of K<sub>b</sub>?</strong> A)CN<sup>-</sup>(aq)+ H<sup>+</sup>(aq) HCN(aq) B)F<sup>-</sup>(aq)+ H<sub>2</sub>O(l) HF(aq)+ OH<sup>-</sup>(aq) C)Zn(OH<sub>2</sub>)<sub>6</sub><sup>2+</sup>(aq) [Zn(OH<sub>2</sub>)<sub>5</sub>OH]<sup>+</sup>(aq)+ H<sup>+</sup>(aq) D)Cr<sup>3+</sup>(aq)+ 6H<sub>2</sub>O(l) Cr(OH<sub>2</sub>)<sub>6</sub><sup>3+</sup>(aq) E)none of these](https://storage.examlex.com/TB2288/11ea7a3a_9f28_1f36_a82d_83558349a513_TB2288_11.jpg) HCN(aq)
HCN(aq)
B)F-(aq)+ H2O(l)![<strong>Which of the following reactions is associated with the definition of K<sub>b</sub>?</strong> A)CN<sup>-</sup>(aq)+ H<sup>+</sup>(aq) HCN(aq) B)F<sup>-</sup>(aq)+ H<sub>2</sub>O(l) HF(aq)+ OH<sup>-</sup>(aq) C)Zn(OH<sub>2</sub>)<sub>6</sub><sup>2+</sup>(aq) [Zn(OH<sub>2</sub>)<sub>5</sub>OH]<sup>+</sup>(aq)+ H<sup>+</sup>(aq) D)Cr<sup>3+</sup>(aq)+ 6H<sub>2</sub>O(l) Cr(OH<sub>2</sub>)<sub>6</sub><sup>3+</sup>(aq) E)none of these](https://storage.examlex.com/TB2288/11ea7a3a_9f28_1f37_a82d_61bc5dd46ef3_TB2288_11.jpg) HF(aq)+ OH-(aq)
HF(aq)+ OH-(aq)
C)Zn(OH2)62+(aq)![<strong>Which of the following reactions is associated with the definition of K<sub>b</sub>?</strong> A)CN<sup>-</sup>(aq)+ H<sup>+</sup>(aq) HCN(aq) B)F<sup>-</sup>(aq)+ H<sub>2</sub>O(l) HF(aq)+ OH<sup>-</sup>(aq) C)Zn(OH<sub>2</sub>)<sub>6</sub><sup>2+</sup>(aq) [Zn(OH<sub>2</sub>)<sub>5</sub>OH]<sup>+</sup>(aq)+ H<sup>+</sup>(aq) D)Cr<sup>3+</sup>(aq)+ 6H<sub>2</sub>O(l) Cr(OH<sub>2</sub>)<sub>6</sub><sup>3+</sup>(aq) E)none of these](https://storage.examlex.com/TB2288/11ea7a3a_9f28_1f38_a82d_1d4e13d6f5ea_TB2288_11.jpg) [Zn(OH2)5OH]+(aq)+ H+(aq)
[Zn(OH2)5OH]+(aq)+ H+(aq)
D)Cr3+(aq)+ 6H2O(l)![<strong>Which of the following reactions is associated with the definition of K<sub>b</sub>?</strong> A)CN<sup>-</sup>(aq)+ H<sup>+</sup>(aq) HCN(aq) B)F<sup>-</sup>(aq)+ H<sub>2</sub>O(l) HF(aq)+ OH<sup>-</sup>(aq) C)Zn(OH<sub>2</sub>)<sub>6</sub><sup>2+</sup>(aq) [Zn(OH<sub>2</sub>)<sub>5</sub>OH]<sup>+</sup>(aq)+ H<sup>+</sup>(aq) D)Cr<sup>3+</sup>(aq)+ 6H<sub>2</sub>O(l) Cr(OH<sub>2</sub>)<sub>6</sub><sup>3+</sup>(aq) E)none of these](https://storage.examlex.com/TB2288/11ea7a3a_9f28_4649_a82d_99ef6625892c_TB2288_11.jpg) Cr(OH2)63+(aq)
Cr(OH2)63+(aq)
E)none of these
A)CN-(aq)+ H+(aq)
![<strong>Which of the following reactions is associated with the definition of K<sub>b</sub>?</strong> A)CN<sup>-</sup>(aq)+ H<sup>+</sup>(aq) HCN(aq) B)F<sup>-</sup>(aq)+ H<sub>2</sub>O(l) HF(aq)+ OH<sup>-</sup>(aq) C)Zn(OH<sub>2</sub>)<sub>6</sub><sup>2+</sup>(aq) [Zn(OH<sub>2</sub>)<sub>5</sub>OH]<sup>+</sup>(aq)+ H<sup>+</sup>(aq) D)Cr<sup>3+</sup>(aq)+ 6H<sub>2</sub>O(l) Cr(OH<sub>2</sub>)<sub>6</sub><sup>3+</sup>(aq) E)none of these](https://storage.examlex.com/TB2288/11ea7a3a_9f28_1f36_a82d_83558349a513_TB2288_11.jpg) HCN(aq)
HCN(aq)B)F-(aq)+ H2O(l)
![<strong>Which of the following reactions is associated with the definition of K<sub>b</sub>?</strong> A)CN<sup>-</sup>(aq)+ H<sup>+</sup>(aq) HCN(aq) B)F<sup>-</sup>(aq)+ H<sub>2</sub>O(l) HF(aq)+ OH<sup>-</sup>(aq) C)Zn(OH<sub>2</sub>)<sub>6</sub><sup>2+</sup>(aq) [Zn(OH<sub>2</sub>)<sub>5</sub>OH]<sup>+</sup>(aq)+ H<sup>+</sup>(aq) D)Cr<sup>3+</sup>(aq)+ 6H<sub>2</sub>O(l) Cr(OH<sub>2</sub>)<sub>6</sub><sup>3+</sup>(aq) E)none of these](https://storage.examlex.com/TB2288/11ea7a3a_9f28_1f37_a82d_61bc5dd46ef3_TB2288_11.jpg) HF(aq)+ OH-(aq)
HF(aq)+ OH-(aq)C)Zn(OH2)62+(aq)
![<strong>Which of the following reactions is associated with the definition of K<sub>b</sub>?</strong> A)CN<sup>-</sup>(aq)+ H<sup>+</sup>(aq) HCN(aq) B)F<sup>-</sup>(aq)+ H<sub>2</sub>O(l) HF(aq)+ OH<sup>-</sup>(aq) C)Zn(OH<sub>2</sub>)<sub>6</sub><sup>2+</sup>(aq) [Zn(OH<sub>2</sub>)<sub>5</sub>OH]<sup>+</sup>(aq)+ H<sup>+</sup>(aq) D)Cr<sup>3+</sup>(aq)+ 6H<sub>2</sub>O(l) Cr(OH<sub>2</sub>)<sub>6</sub><sup>3+</sup>(aq) E)none of these](https://storage.examlex.com/TB2288/11ea7a3a_9f28_1f38_a82d_1d4e13d6f5ea_TB2288_11.jpg) [Zn(OH2)5OH]+(aq)+ H+(aq)
[Zn(OH2)5OH]+(aq)+ H+(aq)D)Cr3+(aq)+ 6H2O(l)
![<strong>Which of the following reactions is associated with the definition of K<sub>b</sub>?</strong> A)CN<sup>-</sup>(aq)+ H<sup>+</sup>(aq) HCN(aq) B)F<sup>-</sup>(aq)+ H<sub>2</sub>O(l) HF(aq)+ OH<sup>-</sup>(aq) C)Zn(OH<sub>2</sub>)<sub>6</sub><sup>2+</sup>(aq) [Zn(OH<sub>2</sub>)<sub>5</sub>OH]<sup>+</sup>(aq)+ H<sup>+</sup>(aq) D)Cr<sup>3+</sup>(aq)+ 6H<sub>2</sub>O(l) Cr(OH<sub>2</sub>)<sub>6</sub><sup>3+</sup>(aq) E)none of these](https://storage.examlex.com/TB2288/11ea7a3a_9f28_4649_a82d_99ef6625892c_TB2288_11.jpg) Cr(OH2)63+(aq)
Cr(OH2)63+(aq)E)none of these

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
43
What is the pH of a 0.35 M solution of methylamine (CH3NH2,Kb = 4.4 × 10-4)at 25oC?
A)5.55
B)0.46
C)12.09
D)13.54
E)1.91
A)5.55
B)0.46
C)12.09
D)13.54
E)1.91

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
44
What is the concentration of C2O42- in a 0.370 M oxalic acid,H2C2O4,solution? For oxalic acid,Ka1 = 5.6 × 10-2 and Ka2 = 5.1 × 10-5.
A)1.2 × 10-1 M
B)-3 × 10-3 M
C)4.3 × 10-3 M
D)5.1 × 10-5 M
E)1.4 × 10-1 M
A)1.2 × 10-1 M
B)-3 × 10-3 M
C)4.3 × 10-3 M
D)5.1 × 10-5 M
E)1.4 × 10-1 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
45
What is the concentration of HC2O4- in a 0.300 M oxalic acid,H2C2O4,solution? For oxalic acid,Ka1 = 5.6 × 10-2 and Ka2 = 5.1 × 10-5.
A)3.9× 10-3 M
B)1.0 × 10-1 M
C)1.3 × 10-1 M
D)3.9 × 10-3 M
E)5.1 × 10-5 M
A)3.9× 10-3 M
B)1.0 × 10-1 M
C)1.3 × 10-1 M
D)3.9 × 10-3 M
E)5.1 × 10-5 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
46
What is the equilibrium concentration of H2C2O4 in a 0.370 M oxalic acid,H2C2O4,solution? For oxalic acid,Ka1 = 5.6 × 10-2 and Ka2 = 5.1 × 10-5.
A)2.5 × 10-1 M
B)1.4 × 10-1 M
C)3.7 × 10-1 M
D)5.1 × 10-5 M
E)1.2 × 10-1 M
A)2.5 × 10-1 M
B)1.4 × 10-1 M
C)3.7 × 10-1 M
D)5.1 × 10-5 M
E)1.2 × 10-1 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
47
A solution of aniline (C6H5NH2,Kb = 4.2 × 10-10)has a pH of 8.56 at 25oC.What was the initial concentration of aniline?
A)2.8 × 10-9 M
B)2.1 × 10-8 M
C)3.2 × 10-2 M
D)4.2 × 10-10 M
E)3.6 × 10-6 M
A)2.8 × 10-9 M
B)2.1 × 10-8 M
C)3.2 × 10-2 M
D)4.2 × 10-10 M
E)3.6 × 10-6 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
48
What is the concentration of HCO3- in a 0.010 M solution of carbonic acid,H2CO3? For carbonic acid,Ka1 = 4.2 × 10-7 and Ka2 = 4.8 × 10-11.
A)6.5 × 10-5 M
B)1.0 × 10-2 M
C)3.2 × 10-5 M
D)4.8 × 10-11 M
E)4.2 × 10-7 M
A)6.5 × 10-5 M
B)1.0 × 10-2 M
C)3.2 × 10-5 M
D)4.8 × 10-11 M
E)4.2 × 10-7 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
49
What is the percent ionization at equilibrium in a 0.10 M solution of dimethylamine,(CH3)2NH (Kb = 5.1 × 10-4),at 25oC?
A)6.8%
B)0.51%
C)0.68%
D)100%
E)10%
A)6.8%
B)0.51%
C)0.68%
D)100%
E)10%

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
50
What is the hydroxide-ion concentration of a 0.150 M oxalic acid,H2C2O4,solution? For oxalic acid,Ka1 = 5.6 × 10-2 and Ka2 = 5.1 × 10-5.
A)3.6 × 10-12 M
B)1.1 × 10-13 M
C)2.7 × 10-3 M
D)1.5 × 10-13 M
E)1.0 × 10-7 M
A)3.6 × 10-12 M
B)1.1 × 10-13 M
C)2.7 × 10-3 M
D)1.5 × 10-13 M
E)1.0 × 10-7 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
51
For which of the following equilibria does Kc correspond to the base-ionization constant,Kb,of HCO3-?
A)HCO3-(aq)+ H2O(l) CO32-(aq)+ H3O+(aq)
CO32-(aq)+ H3O+(aq)
B)HCO3-(aq)+ H2O(l) H2CO3(aq)+ OH-(aq)
H2CO3(aq)+ OH-(aq)
C)H2CO3(aq)+ H2O(l) HCO3-(aq)+ H3O+(aq)
HCO3-(aq)+ H3O+(aq)
D)HCO3-(aq)+ OH-(aq) CO32-(aq)+ H2O(l)
CO32-(aq)+ H2O(l)
E)HCO3-(aq)+ H3O+(aq) H2CO3(aq)+ H2O(l)
H2CO3(aq)+ H2O(l)
A)HCO3-(aq)+ H2O(l)
 CO32-(aq)+ H3O+(aq)
CO32-(aq)+ H3O+(aq)B)HCO3-(aq)+ H2O(l)
 H2CO3(aq)+ OH-(aq)
H2CO3(aq)+ OH-(aq)C)H2CO3(aq)+ H2O(l)
 HCO3-(aq)+ H3O+(aq)
HCO3-(aq)+ H3O+(aq)D)HCO3-(aq)+ OH-(aq)
 CO32-(aq)+ H2O(l)
CO32-(aq)+ H2O(l)E)HCO3-(aq)+ H3O+(aq)
 H2CO3(aq)+ H2O(l)
H2CO3(aq)+ H2O(l)
Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
52
What is the hydronium-ion concentration at equilibrium in a 0.10 M solution of aniline (C6H5NH2,Kb = 4.2 × 10-10)at 25oC?
A)1.0 × 10-13 M
B)1.0 × 10-1 M
C)1.5 × 10-9 M
D)6.5 × 10-6 M
E)6.5 × 10-5 M
A)1.0 × 10-13 M
B)1.0 × 10-1 M
C)1.5 × 10-9 M
D)6.5 × 10-6 M
E)6.5 × 10-5 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
53
What is the equilibrium concentration of ammonium ion in a 0.33 M solution of ammonia (NH3,Kb = 1.8 × 10-5)at 25oC?
A)3.0 × 10-14 M
B)4.1 × 10-12 M
C)2.4 × 10-3 M
D)7.4 × 10-3 M
E)3.3 × 10-1 M
A)3.0 × 10-14 M
B)4.1 × 10-12 M
C)2.4 × 10-3 M
D)7.4 × 10-3 M
E)3.3 × 10-1 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
54
What is the hydroxide-ion concentration at equilibrium in a 0.66 M solution of ethylamine (C2H5NH2,Kb = 4.7 × 10-4)at 25oC?
A)1.7 × 10-2 M
B)2.7 × 10-2 M
C)1.5 × 10-14 M
D)5.8 × 10-13 M
E)6.6 × 10-1 M
A)1.7 × 10-2 M
B)2.7 × 10-2 M
C)1.5 × 10-14 M
D)5.8 × 10-13 M
E)6.6 × 10-1 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
55
What is the pOH of a 0.17 M solution of pyridine (Kb = 1.4 × 10-9)at 25°C?
A)1.54
B)4.04
C)8.85
D)4.81
E)11.08
A)1.54
B)4.04
C)8.85
D)4.81
E)11.08

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
56
For which of the following equilibria does Kc correspond to a base-ionization constant,Kb?
A)H3O+(aq)+ OH-(aq) 2H2O(l)
2H2O(l)
B)HCO3-(aq)+ OH-(aq) CO32-(aq)+ H2O(l)
CO32-(aq)+ H2O(l)
C)HOCl(aq)+ H2O(l) H3O+(aq)+ OCl-(aq)
H3O+(aq)+ OCl-(aq)
D)CO32-(aq)+ H2O(l) HCO3-(aq)+ OH-(aq)
HCO3-(aq)+ OH-(aq)
E)HCHO2(aq)+ NH3(aq) CHO2-(aq)+ NH4+(aq)
CHO2-(aq)+ NH4+(aq)
A)H3O+(aq)+ OH-(aq)
 2H2O(l)
2H2O(l)B)HCO3-(aq)+ OH-(aq)
 CO32-(aq)+ H2O(l)
CO32-(aq)+ H2O(l)C)HOCl(aq)+ H2O(l)
 H3O+(aq)+ OCl-(aq)
H3O+(aq)+ OCl-(aq)D)CO32-(aq)+ H2O(l)
 HCO3-(aq)+ OH-(aq)
HCO3-(aq)+ OH-(aq)E)HCHO2(aq)+ NH3(aq)
 CHO2-(aq)+ NH4+(aq)
CHO2-(aq)+ NH4+(aq)
Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
57
The equilibrium hydronium ion concentration of an initially 0.510 M solution of a diprotic weak acid (H2A)is 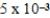 M.What is Ka1? (assume Ca/Ka ≥ 102 and Ka1 >> Ka2)
M.What is Ka1? (assume Ca/Ka ≥ 102 and Ka1 >> Ka2)
A)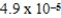
B)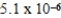
C)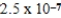
D)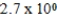
E)not enough information provided
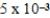 M.What is Ka1? (assume Ca/Ka ≥ 102 and Ka1 >> Ka2)
M.What is Ka1? (assume Ca/Ka ≥ 102 and Ka1 >> Ka2)A)
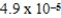
B)
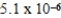
C)
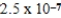
D)
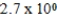
E)not enough information provided

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
58
What is the hydronium-ion concentration in a 0.018 M solution of carbonic acid,H2CO3? For carbonic acid,Ka1 = 4.2 × 10-7 and Ka2 = 4.8 × 10-11.
A)1.8 × 10-2 M
B)4.2 × 10-7 M
C)4.8 × 10-11 M
D)8.7 × 10-5 M
E)4.3 × 10-5 M
A)1.8 × 10-2 M
B)4.2 × 10-7 M
C)4.8 × 10-11 M
D)8.7 × 10-5 M
E)4.3 × 10-5 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
59
What is the concentration of CO32- in a 0.033 M solution of carbonic acid,H2CO3? For carbonic acid,Ka1 = 4.2 × 10-7 and Ka2 = 4.8 × 10-11.
A)3.3 × 10-2 M
B)1.2 × 10-4 M
C)5.9 × 10-5 M
D)4.8 × 10-11 M
E)4.2 × 10-7 M
A)3.3 × 10-2 M
B)1.2 × 10-4 M
C)5.9 × 10-5 M
D)4.8 × 10-11 M
E)4.2 × 10-7 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
60
What is the base-ionization equilibrium constant for an aqueous solution of ammonia,NH3?
A)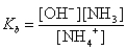
B)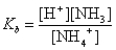
C)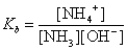
D)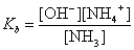
E)none of these
A)
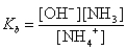
B)
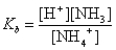
C)
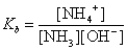
D)
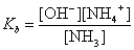
E)none of these

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following equilibria best represents the hydrolysis reaction that occurs in an aqueous solution of NH4Cl?
A)Cl-(aq)+ H3O+(aq) HCl(aq)+ H2O(l)
HCl(aq)+ H2O(l)
B)NH4+(aq)+ H2O(l) NH3(aq)+ H3O+(aq)
NH3(aq)+ H3O+(aq)
C)NH4+(aq)+ OH-(aq) NH3(aq)+ H2O(l)
NH3(aq)+ H2O(l)
D)Cl-(aq)+ H2O(l) HCl(aq)+ OH-(aq)
HCl(aq)+ OH-(aq)
E)NH4+(aq)+ Cl-(aq) NH4Cl(s)
NH4Cl(s)
A)Cl-(aq)+ H3O+(aq)
 HCl(aq)+ H2O(l)
HCl(aq)+ H2O(l)B)NH4+(aq)+ H2O(l)
 NH3(aq)+ H3O+(aq)
NH3(aq)+ H3O+(aq)C)NH4+(aq)+ OH-(aq)
 NH3(aq)+ H2O(l)
NH3(aq)+ H2O(l)D)Cl-(aq)+ H2O(l)
 HCl(aq)+ OH-(aq)
HCl(aq)+ OH-(aq)E)NH4+(aq)+ Cl-(aq)
 NH4Cl(s)
NH4Cl(s)
Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
62
Saccharin is a weak organic base with a Kb of 4.80 × 10-3.A 0.297-g sample of saccharin dissolved in 25.0 mL of water has a pH of 12.190.What is the molar mass of saccharin?
A)0.823 g/mol
B)33.700 g/mol
C)6.824090785E+24 g/mol
D)185 g/mol
E)278 g/mol
A)0.823 g/mol
B)33.700 g/mol
C)6.824090785E+24 g/mol
D)185 g/mol
E)278 g/mol

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
63
What is Kb for the following equilibrium? Ka for HNO2 is 5.0 × 10-4.
NO2-(aq)+ H2O(l)
HNO2(aq)+ OH-(aq)
A)2.0 × 10-4
B)5.0 × 10-4
C)5.0 × 1010
D)5.0 × 1018
E)2.0 × 10-11
NO2-(aq)+ H2O(l)

HNO2(aq)+ OH-(aq)
A)2.0 × 10-4
B)5.0 × 10-4
C)5.0 × 1010
D)5.0 × 1018
E)2.0 × 10-11

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following salts is most likely to form an aqueous solution having the pH shown in the figure below? 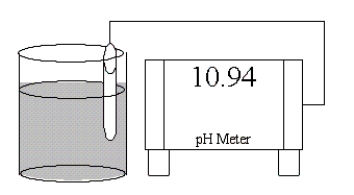
A)KCl
B)Zn(NO3)2
C)NaCN
D)NH4Cl
E)LiBr

A)KCl
B)Zn(NO3)2
C)NaCN
D)NH4Cl
E)LiBr

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
65
The Ka for hydrofluoric acid is 6.8 × 10-4.What is Kb for the fluoride ion?
A)1.5 × 10-11
B)6.8 × 10-4
C)1.5 × 103
D)6.8 × 1010
E)6.8 × 10-18
A)1.5 × 10-11
B)6.8 × 10-4
C)1.5 × 103
D)6.8 × 1010
E)6.8 × 10-18

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
66
Consider the reaction NH3(aq)+ H2O(l)  NH4+(aq)+ OH-(aq).Kb for NH3 is 1.8 × 10-5 at 25°C.What is Ka for the NH4+ ion at 25°C?
NH4+(aq)+ OH-(aq).Kb for NH3 is 1.8 × 10-5 at 25°C.What is Ka for the NH4+ ion at 25°C?
A)5.6 × 104
B)5.6 × 10-10
C)1.8 × 10-5
D)7.2 × 10-12
E)9.2 × 10-8
 NH4+(aq)+ OH-(aq).Kb for NH3 is 1.8 × 10-5 at 25°C.What is Ka for the NH4+ ion at 25°C?
NH4+(aq)+ OH-(aq).Kb for NH3 is 1.8 × 10-5 at 25°C.What is Ka for the NH4+ ion at 25°C?A)5.6 × 104
B)5.6 × 10-10
C)1.8 × 10-5
D)7.2 × 10-12
E)9.2 × 10-8

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following salts is most likely to form an aqueous solution having the pH shown in the figure below? 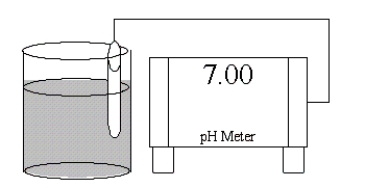
A)Na2CO3
B)RbF
C)NH4Cl
D)Zn(NO3)2
E)KCl

A)Na2CO3
B)RbF
C)NH4Cl
D)Zn(NO3)2
E)KCl

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
68
What is Ka at 25°C for the following equilibrium?
CH3NH3+(aq)+ H2O(l)
CH3NH2(aq)+ H3O+(aq)
Kb (CH3NH2)= 4.4 × 10-4 at 25°C.
A)4.4 × 10-4
B)2.3 × 103
C)4.4 × 10-10
D)4.4 × 104
E)2.3 × 10-11
CH3NH3+(aq)+ H2O(l)

CH3NH2(aq)+ H3O+(aq)
Kb (CH3NH2)= 4.4 × 10-4 at 25°C.
A)4.4 × 10-4
B)2.3 × 103
C)4.4 × 10-10
D)4.4 × 104
E)2.3 × 10-11

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following salts is most likely to form an aqueous solution having the pH shown in the figure below? 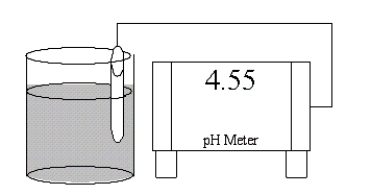
A)NH4Cl
B)NaBr
C)K2CO3
D)RbCN
E)LiNO3

A)NH4Cl
B)NaBr
C)K2CO3
D)RbCN
E)LiNO3

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following statements is true concerning a 0.1 M solution of Na2S and a 0.1 M solution of NaHS? For H2S,Ka1 = 1.0 × 10-7 and Ka2 = 1.3 × 10-13.
A)Both solutions are neutral.
B)The sodium hydrogen sulfide solution is the more basic.
C)Both solutions have the same pH.
D)The sodium sulfide solution is the more basic.
E)Both the solutions are acidic.
A)Both solutions are neutral.
B)The sodium hydrogen sulfide solution is the more basic.
C)Both solutions have the same pH.
D)The sodium sulfide solution is the more basic.
E)Both the solutions are acidic.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following solutions has the highest hydroxide-ion concentration?
A)0.10 M NH4ClO4
B)0.10 M NaI
C)0.10 M NaNO3
D)0.10 M NH4Cl
E)0.10 M NaCN
A)0.10 M NH4ClO4
B)0.10 M NaI
C)0.10 M NaNO3
D)0.10 M NH4Cl
E)0.10 M NaCN

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
72
Given the following,what will be the approximate equilibrium pH of an aqueous solution of ammonium cyanide,NH4CN?
NH4+
Ka = 5.69 x10-10
HCN
Ka = 6.2 x10-10
A)slightly basic
B)slightly acidic
C)nearly neutral
NH4+
Ka = 5.69 x10-10
HCN
Ka = 6.2 x10-10
A)slightly basic
B)slightly acidic
C)nearly neutral

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
73
Given the following,what will be the approximate equilibrium pH of an aqueous solution of ammonium acetate,NH4CH3CO2?
NH4+
Ka = 5.69 x10-10
CH3CO2-
Kb = 5.71 x10-10
A)very basic
B)very acidic
C)nearly neutral
NH4+
Ka = 5.69 x10-10
CH3CO2-
Kb = 5.71 x10-10
A)very basic
B)very acidic
C)nearly neutral

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following salts will produce a neutral solution when added to pure water?
A)LiHCO3
B)NH4NO3
C)Na2SO4
D)RbNO2
E)KNO3
A)LiHCO3
B)NH4NO3
C)Na2SO4
D)RbNO2
E)KNO3

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
75
The amphiprotic anion monohydrogenphosphate acts as both an acid and a base in aqueous solution.Given the following acid-ionization and base-hydrolysis constants,what will be the approximate equilibrium pH of an aqueous solution of sodium monohydrogenphosphate?
Ka =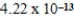
Kb =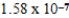
A)basic
B)acidic
C)neutral
Ka =
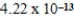
Kb =
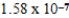
A)basic
B)acidic
C)neutral

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
76
A 0.0678 M solution of a weak base has a pH of 9.44.What is the identity of the weak base?
Weak Base
Kb
Ethylamine (CH3CH2NH2)
4)7 × 10-4
Hydrazine (N2H4)
1)7 × 10-6
Hydroxylamine (NH2OH)
1)1 × 10-8
Pyridine (C5H5N)
1)4 × 10-9
Aniline (C6H5NH2)
4)2 × 10-10
A)hydrazine
B)pyridine
C)ethylamine
D)hydroxylamine
E)aniline
Weak Base
Kb
Ethylamine (CH3CH2NH2)
4)7 × 10-4
Hydrazine (N2H4)
1)7 × 10-6
Hydroxylamine (NH2OH)
1)1 × 10-8
Pyridine (C5H5N)
1)4 × 10-9
Aniline (C6H5NH2)
4)2 × 10-10
A)hydrazine
B)pyridine
C)ethylamine
D)hydroxylamine
E)aniline

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
77
What is Ka for the anilinium cation,C6H5NH3+,at 25°C? (Kb for C6H5NH2 = 4.2 × 10-10 at 25°C.)
A)1.0 × 10-7
B)4.2 × 104
C)4.2 × 10-24
D)2.4 × 10-5
E)4.2 × 10-10
A)1.0 × 10-7
B)4.2 × 104
C)4.2 × 10-24
D)2.4 × 10-5
E)4.2 × 10-10

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following salts forms an acidic aqueous solution?
A)NaNO2
B)KCH3CO2
C)Rb2O
D)FeCl3
E)NaCN
A)NaNO2
B)KCH3CO2
C)Rb2O
D)FeCl3
E)NaCN

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following salts will produce an acidic solution when added to pure water?
A)Cs2CO3
B)NaF
C)KCN
D)Al(NO3)3
E)Li2S
A)Cs2CO3
B)NaF
C)KCN
D)Al(NO3)3
E)Li2S

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following equilibria best represents the hydrolysis reaction that occurs in an aqueous solution of KNO2?
A)NO2-(aq)+ H2O(l) HNO3(aq)+ 2H+(aq)
HNO3(aq)+ 2H+(aq)
B)NO2-(aq)+ H2O(l) HNO2(aq)+ OH-(aq)
HNO2(aq)+ OH-(aq)
C)NO2-(aq)+ H3O+(aq) HNO2(aq)+ H2O(l)
HNO2(aq)+ H2O(l)
D)K+(aq)+ NO2-(aq)+ H2O(l) KOH(aq)+ HNO2(aq)
KOH(aq)+ HNO2(aq)
E)K+(aq)+ H2O(l) KOH(aq)+ H+(aq)
KOH(aq)+ H+(aq)
A)NO2-(aq)+ H2O(l)
 HNO3(aq)+ 2H+(aq)
HNO3(aq)+ 2H+(aq)B)NO2-(aq)+ H2O(l)
 HNO2(aq)+ OH-(aq)
HNO2(aq)+ OH-(aq)C)NO2-(aq)+ H3O+(aq)
 HNO2(aq)+ H2O(l)
HNO2(aq)+ H2O(l)D)K+(aq)+ NO2-(aq)+ H2O(l)
 KOH(aq)+ HNO2(aq)
KOH(aq)+ HNO2(aq)E)K+(aq)+ H2O(l)
 KOH(aq)+ H+(aq)
KOH(aq)+ H+(aq)
Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck


