Exam 16: Acid-Base Equilibria
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
What is the hydroxide-ion concentration of a 0.190 M sodium oxalate (Na2C2O4)solution? For oxalic acid (H2C2O4),Ka1 = 5.6 × 10-2 and Ka2 = 5.1 × 10-5.
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
A
Calculate the pH of a solution that is 2.00 M HF,1.00 M NaOH,and 0.546 M NaF.(Ka = 6.8 × 10-4)
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
A
What is Ka for the anilinium cation,C6H5NH3+,at 25°C? (Kb for C6H5NH2 = 4.2 × 10-10 at 25°C.)
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
D
What is the hydronium-ion concentration in a solution resulting from mixing 133 mL of 0.100 M HCN and 80 mL of 0.100 M KOH at 25°C? Ka for HCN = 4.9 × 10-10 at 25°C.
(Multiple Choice)
4.9/5  (33)
(33)
For a 0.10 M solution of glutaric acid,HO2C(CH2)3CO2H (Ka1 = 4.6 × 10-5,Ka2 = 3.9 × 10-6),rank the following species in order of increasing equilibrium concentration.
(Multiple Choice)
4.8/5  (28)
(28)
Which of the following salts is most likely to form an aqueous solution having the pH shown in the figure below? 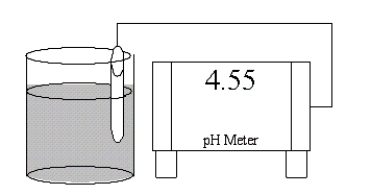
(Multiple Choice)
4.8/5  (31)
(31)
Saccharin is a weak organic base with a Kb of 4.80 × 10-3.A 0.297-g sample of saccharin dissolved in 25.0 mL of water has a pH of 12.190.What is the molar mass of saccharin?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following solutions will not yield an effective NaH2PO4/Na2HPO4 buffer?
(Multiple Choice)
4.9/5  (38)
(38)
Which acid-base combination is depicted by this titration curve? 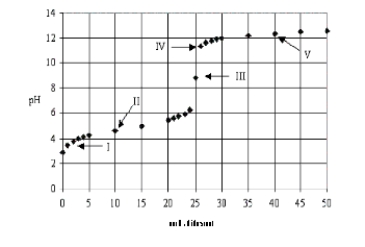
(Multiple Choice)
4.8/5  (31)
(31)
What will happen if a small amount of sodium hydroxide is added to a 0.1 M solution of ammonia?
(Multiple Choice)
4.8/5  (32)
(32)
Suppose a buffer solution is made from formic acid (HCHO2)and sodium formate (NaCHO2).What is the net ionic equation for the reaction that occurs when a small amount of hydrochloric acid is added to the buffer?
(Multiple Choice)
4.7/5  (35)
(35)
Which of the following solutions would show the greatest change in pH upon the addition of 10.0 mL of 1.0 M NaOH to 1.0 L of the solution?
(Multiple Choice)
4.8/5  (35)
(35)
What is the pH of a 0.35 M solution of methylamine (CH3NH2,Kb = 4.4 × 10-4)at 25oC?
(Multiple Choice)
4.7/5  (44)
(44)
Which of the following salts is most likely to form an aqueous solution having the pH shown in the figure below? 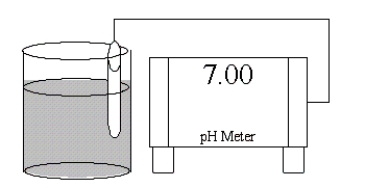
(Multiple Choice)
4.8/5  (31)
(31)
What is the concentration of C2O42- in a 0.370 M oxalic acid,H2C2O4,solution? For oxalic acid,Ka1 = 5.6 × 10-2 and Ka2 = 5.1 × 10-5.
(Multiple Choice)
4.9/5  (41)
(41)
For a solution equimolar in HCN and NaCN,which statement is false?
(Multiple Choice)
4.9/5  (27)
(27)
Which of the following is the most effective buffer system for a pH value of 4.45?
(Multiple Choice)
4.9/5  (31)
(31)
What is the hydronium-ion concentration at equilibrium in a 0.10 M solution of aniline (C6H5NH2,Kb = 4.2 × 10-10)at 25oC?
(Multiple Choice)
4.8/5  (38)
(38)
In a 0.01 M solution of 1,4-butanedicarboxylic acid,HOOCCH2CH2COOH (Ka1 = 2.9 × 10-5,Ka2 = 5.3 × 10-6),which species is present in the lowest concentration?
(Multiple Choice)
4.8/5  (26)
(26)
What is the pH of an initially 0.428 M solution of a weak monoprotic acid that is 0.11 % ionized when equilibrium is established? (assume Ca/Ka ≥102)
(Multiple Choice)
4.8/5  (27)
(27)
Showing 1 - 20 of 148
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)