Deck 17: Solubility and Complex-Ion Equilibria
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/115
Play
Full screen (f)
Deck 17: Solubility and Complex-Ion Equilibria
1
What is the correct mathematical expression for finding the molar solubility (s)of Sn(OH)2?
A)2s3 = Ksp
B)4s3 = Ksp
C)108s5 = Ksp
D)2s2 = Ksp
E)8s3 = Ksp
A)2s3 = Ksp
B)4s3 = Ksp
C)108s5 = Ksp
D)2s2 = Ksp
E)8s3 = Ksp
4s3 = Ksp
2
What is the solubility product expression for Pb(IO3)2?
A)Ksp = [Pb2+][IO3-]2
B)Ksp = [Pb4+][2IO32-]2
C)Ksp = [Pb2+][2IO3-]
D)Ksp = [Pb4+][IO32-]2
E)Ksp = [Pb2+][2IO3-]2
A)Ksp = [Pb2+][IO3-]2
B)Ksp = [Pb4+][2IO32-]2
C)Ksp = [Pb2+][2IO3-]
D)Ksp = [Pb4+][IO32-]2
E)Ksp = [Pb2+][2IO3-]2
Ksp = [Pb2+][IO3-]2
3
What is the solubility product expression for Zn3(PO4)2?
A)Ksp = [Zn32+][(PO43-)2]
B)Ksp = [3Zn2+]3[2PO43-]2
C)Ksp = [Zn2+][2PO43-]
D)Ksp = [Zn3+]2[PO42-]3
E)Ksp = [Zn2+]3[PO43-]2
A)Ksp = [Zn32+][(PO43-)2]
B)Ksp = [3Zn2+]3[2PO43-]2
C)Ksp = [Zn2+][2PO43-]
D)Ksp = [Zn3+]2[PO42-]3
E)Ksp = [Zn2+]3[PO43-]2
Ksp = [Zn2+]3[PO43-]2
4
Figures I-IV represent ionic compounds formed upon the mixing of an aqueous solution containing cation C with an aqueous solution containing anion A.Identify the figure(s)that represent(s)products for which Ksp = 108s5,where s is the molar solubility of the ionic compound. 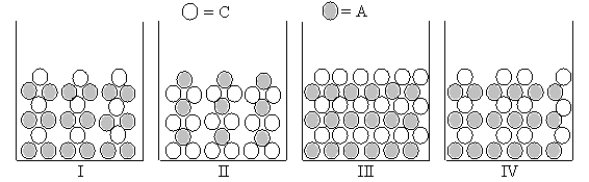
A)only II
B)both I and II
C)only IV
D)only III
E)only I

A)only II
B)both I and II
C)only IV
D)only III
E)only I

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
5
Cation C and anion A form an ionic compound for which Ksp = 4s3,where s is the molar solubility of the ionic compound.Which of Figures I-III represent(s)possible results of the mixing of an aqueous solution containing cation C with an aqueous solution containing anion A? 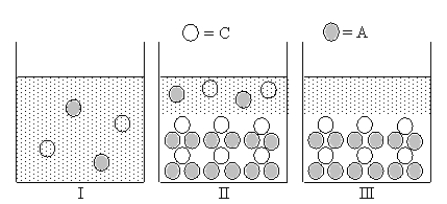
A)only III
B)only II
C)both I and II
D)only I
E)both I and III

A)only III
B)only II
C)both I and II
D)only I
E)both I and III

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
6
The concentration of barium carbonate in a saturated aqueous solution of the salt at 25°C is 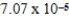 M.What is the Ksp of this sparingly soluble salt?
M.What is the Ksp of this sparingly soluble salt?
A)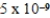
B)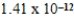
C)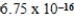
D)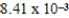
E)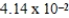
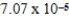 M.What is the Ksp of this sparingly soluble salt?
M.What is the Ksp of this sparingly soluble salt?A)
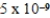
B)
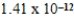
C)
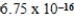
D)
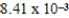
E)
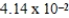

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
7
What is the solubility product expression for Al(OH)3?
A)Ksp = [Al3+][3OH-]
B)Ksp = 3[Al3+][OH-]3
C)Ksp = [Al3+][OH-]3
D)Ksp = [Al3+][3OH-]3
E)Ksp = [Al3+][OH-]
A)Ksp = [Al3+][3OH-]
B)Ksp = 3[Al3+][OH-]3
C)Ksp = [Al3+][OH-]3
D)Ksp = [Al3+][3OH-]3
E)Ksp = [Al3+][OH-]

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
8
After mixing an excess PbCl2 with a fixed amount of water,it is found that the equilibrium concentration of Pb2+ is 1.6 × 10-2 M.What is Ksp for PbCl2?
A)4.0 × 10-6
B)1.6 × 10-5
C)2.5 × 10-4
D)4.8 × 10-2
E)1.0 × 10-6
A)4.0 × 10-6
B)1.6 × 10-5
C)2.5 × 10-4
D)4.8 × 10-2
E)1.0 × 10-6

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
9
The solubility of calcium carbonate in water at 25°C is 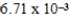 g/L.What is the Ksp of this sparingly soluble salt?
g/L.What is the Ksp of this sparingly soluble salt?
A)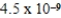
B)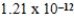
C)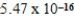
D)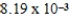
E)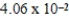
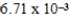 g/L.What is the Ksp of this sparingly soluble salt?
g/L.What is the Ksp of this sparingly soluble salt?A)
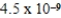
B)
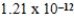
C)
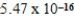
D)
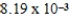
E)
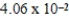

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
10
The solubility of lead(II)sulfate is 4.0 × 10-2 g/L.What is the solubility product constant for lead(II)sulfate?
A)1.7 × 10-8
B)1.3 × 10-4
C)1.6 × 10-3
D)4.6× 10-15
E)8.9 × 10-12
A)1.7 × 10-8
B)1.3 × 10-4
C)1.6 × 10-3
D)4.6× 10-15
E)8.9 × 10-12

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
11
What is the solubility product expression for La2(CO3)3?
A)Ksp = [2La3+]2[3CO32-]3
B)Ksp = [La2+]2[CO32-]3
C)Ksp = [2La3+]2[CO32-]3
D)Ksp = [2La3+][3CO32-]
E)Ksp = [La3+]2[CO32-]3
A)Ksp = [2La3+]2[3CO32-]3
B)Ksp = [La2+]2[CO32-]3
C)Ksp = [2La3+]2[CO32-]3
D)Ksp = [2La3+][3CO32-]
E)Ksp = [La3+]2[CO32-]3

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
12
Figures I-IV represent ionic compounds formed upon the mixing of an aqueous solution containing cation C with an aqueous solution containing anion A.Identify the figure(s)that represent(s)products for which Ksp = s2,where s is the molar solubility of the ionic compound. 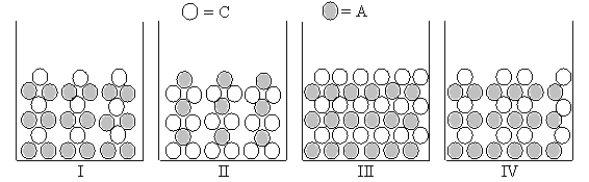
A)only I
B)only II
C)only IV
D)only III
E)both I and II

A)only I
B)only II
C)only IV
D)only III
E)both I and II

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
13
What is the relationship between molar solubility (s)and Ksp for calcium fluoride?
A)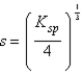
B)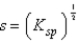
C)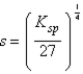
D)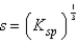
E)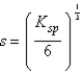
A)
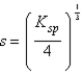
B)
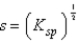
C)
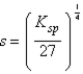
D)
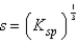
E)
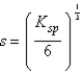

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
14
What is the solubility product expression for mercury(I)chloride,Hg2Cl2?
A)Ksp = [Hg22+][2Cl-]2
B)Ksp = [Hg22+][Cl-]2
C)Ksp = [Hg22+][2Cl- ]
D)Ksp = [Hg2][Cl2]
E)Ksp = [Hg+]2[Cl-]2
A)Ksp = [Hg22+][2Cl-]2
B)Ksp = [Hg22+][Cl-]2
C)Ksp = [Hg22+][2Cl- ]
D)Ksp = [Hg2][Cl2]
E)Ksp = [Hg+]2[Cl-]2

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
15
The solubility of silver(I)carbonate is 3.6 × 10-2 g/L.What is the solubility product constant for silver(I)carbonate?
A)4.4 × 10-15
B)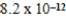
C)1.7 × 10-8
D)1.3 × 10-4
E)1.3 × 10-3
A)4.4 × 10-15
B)
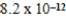
C)1.7 × 10-8
D)1.3 × 10-4
E)1.3 × 10-3

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
16
What is the solubility product expression for Tb3(PO4)4?
A)Ksp = [Tb3+]4[PO44-]3
B)Ksp = [3Tb3+][4PO43-]
C)Ksp = [3Tb3+]3[4PO43-]4
D)Ksp = [Tb2+]3[PO43-]2
E)Ksp = [Tb4+]3[PO43-]4
A)Ksp = [Tb3+]4[PO44-]3
B)Ksp = [3Tb3+][4PO43-]
C)Ksp = [3Tb3+]3[4PO43-]4
D)Ksp = [Tb2+]3[PO43-]2
E)Ksp = [Tb4+]3[PO43-]4

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
17
Cation C and anion A form an ionic compound for which Ksp = s2,where s is the molar solubility of the ionic compound.Which of Figures I-III represent(s)possible results of the mixing of an aqueous solution containing cation C with an aqueous solution containing anion A? 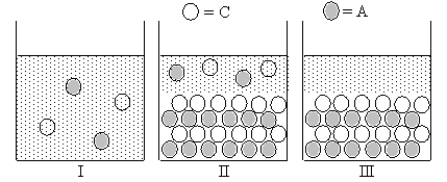
A)only I
B)only III
C)both I and III
D)both I and II
E)only II

A)only I
B)only III
C)both I and III
D)both I and II
E)only II

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
18
Figures I-IV represent ionic compounds formed upon the mixing of an aqueous solution containing cation C with an aqueous solution containing anion A.Identify the figure(s)that represent(s)products for which Ksp = 4s3,where s is the molar solubility of the ionic compound. 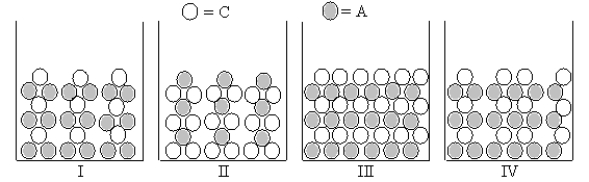
A)both I and II
B)only II
C)only IV
D)only I
E)only III

A)both I and II
B)only II
C)only IV
D)only I
E)only III

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
19
What is the solubility product expression for Pb(IO3)4?
A)Ksp = [Pb4+][4IO3-]4
B)Ksp = [Pb4+][IO3-]
C)Ksp = [Pb][IO3]4
D)Ksp = [Pb4+][IO3-]4
A)Ksp = [Pb4+][4IO3-]4
B)Ksp = [Pb4+][IO3-]
C)Ksp = [Pb][IO3]4
D)Ksp = [Pb4+][IO3-]4

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following particulate views is/are consistent with a heterogeneous equilibrium? 


I
II
III
A)I only
B)II only
C)III only
D)II and III
E)I,II,and III



I
II
III
A)I only
B)II only
C)III only
D)II and III
E)I,II,and III

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following salts has the lowest molar solubility?
A)SrCO3 (Ksp = 9.3 × 10-10)
B)MnS (Ksp = 2.5 × 10-10)
C)BaF2 (Ksp = 1 × 10-6)
D)BaSO4 (Ksp = 1.1 × 10-10)
E)AgCl (Ksp = 1.8 × 10-10)
A)SrCO3 (Ksp = 9.3 × 10-10)
B)MnS (Ksp = 2.5 × 10-10)
C)BaF2 (Ksp = 1 × 10-6)
D)BaSO4 (Ksp = 1.1 × 10-10)
E)AgCl (Ksp = 1.8 × 10-10)

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
22
What is the molar solubility of lead(II)sulfate at 25°C? The solubility product constant for lead(II)sulfate is 1.7 × 10-8 at 25°C.
A)1.7 × 10-8 M
B)5.7 × 10-3 M
C)8.5 × 10-9 M
D)1.6 × 10-3 M
E)1.3 × 10-4 M
A)1.7 × 10-8 M
B)5.7 × 10-3 M
C)8.5 × 10-9 M
D)1.6 × 10-3 M
E)1.3 × 10-4 M

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
23
Pure water is saturated with slightly soluble calcium fluoride,CaF2.Which of the following is true concerning the equilibrium concentration of Ca2+?
A)![<strong>Pure water is saturated with slightly soluble calcium fluoride,CaF<sub>2</sub>.Which of the following is true concerning the equilibrium concentration of Ca<sup>2+</sup>?</strong> A) B)[Ca<sup>2+</sup>] = [F<sup>-</sup>] C) D) E)[Ca<sup>2+</sup>] = K<sub>sp</sub>](https://storage.examlex.com/TB2288/11ea7a3a_9f30_f91a_a82d_8d61a9ee3ff1_TB2288_11.jpg)
B)[Ca2+] = [F-]
C)![<strong>Pure water is saturated with slightly soluble calcium fluoride,CaF<sub>2</sub>.Which of the following is true concerning the equilibrium concentration of Ca<sup>2+</sup>?</strong> A) B)[Ca<sup>2+</sup>] = [F<sup>-</sup>] C) D) E)[Ca<sup>2+</sup>] = K<sub>sp</sub>](https://storage.examlex.com/TB2288/11ea7a3a_9f30_f91b_a82d_7710277ae6fe_TB2288_11.jpg)
D)![<strong>Pure water is saturated with slightly soluble calcium fluoride,CaF<sub>2</sub>.Which of the following is true concerning the equilibrium concentration of Ca<sup>2+</sup>?</strong> A) B)[Ca<sup>2+</sup>] = [F<sup>-</sup>] C) D) E)[Ca<sup>2+</sup>] = K<sub>sp</sub>](https://storage.examlex.com/TB2288/11ea7a3a_9f30_f91c_a82d_51386b021931_TB2288_11.jpg)
E)[Ca2+] = Ksp
A)
![<strong>Pure water is saturated with slightly soluble calcium fluoride,CaF<sub>2</sub>.Which of the following is true concerning the equilibrium concentration of Ca<sup>2+</sup>?</strong> A) B)[Ca<sup>2+</sup>] = [F<sup>-</sup>] C) D) E)[Ca<sup>2+</sup>] = K<sub>sp</sub>](https://storage.examlex.com/TB2288/11ea7a3a_9f30_f91a_a82d_8d61a9ee3ff1_TB2288_11.jpg)
B)[Ca2+] = [F-]
C)
![<strong>Pure water is saturated with slightly soluble calcium fluoride,CaF<sub>2</sub>.Which of the following is true concerning the equilibrium concentration of Ca<sup>2+</sup>?</strong> A) B)[Ca<sup>2+</sup>] = [F<sup>-</sup>] C) D) E)[Ca<sup>2+</sup>] = K<sub>sp</sub>](https://storage.examlex.com/TB2288/11ea7a3a_9f30_f91b_a82d_7710277ae6fe_TB2288_11.jpg)
D)
![<strong>Pure water is saturated with slightly soluble calcium fluoride,CaF<sub>2</sub>.Which of the following is true concerning the equilibrium concentration of Ca<sup>2+</sup>?</strong> A) B)[Ca<sup>2+</sup>] = [F<sup>-</sup>] C) D) E)[Ca<sup>2+</sup>] = K<sub>sp</sub>](https://storage.examlex.com/TB2288/11ea7a3a_9f30_f91c_a82d_51386b021931_TB2288_11.jpg)
E)[Ca2+] = Ksp

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
24
What is the solubility (in g/L)of barium chromate at 25°C? The solubility product constant for barium chromate is 1.2 × 10-10 at 25°C.
A)0.41 g/L
B)3.0 × 10-8 g/L
C)1.5 × 10-8 g/L
D)0.078 g/L
E)0.0027 g/L
A)0.41 g/L
B)3.0 × 10-8 g/L
C)1.5 × 10-8 g/L
D)0.078 g/L
E)0.0027 g/L

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
25
A saturated solution of which of the following salts will have the highest molar concentration of chromate ion?
A)CuCrO4 (Ksp = 3.6 × 10-6)
B)BaCrO4 (Ksp = 2.1 × 10-10)
C)Ag2CrO4 (Ksp = 1.2 × 10-12)
D)Hg2CrO4 (Ksp = 2.0 × 10-9)
E)Tl2CrO4 (Ksp = 9.8 × 10-13)
A)CuCrO4 (Ksp = 3.6 × 10-6)
B)BaCrO4 (Ksp = 2.1 × 10-10)
C)Ag2CrO4 (Ksp = 1.2 × 10-12)
D)Hg2CrO4 (Ksp = 2.0 × 10-9)
E)Tl2CrO4 (Ksp = 9.8 × 10-13)

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
26
What is the solubility (in g/L)of aluminum hydroxide at 25°C? The solubility product constant for aluminum hydroxide is 4.6× 10-33 at 25°C.
A)3.6 × 10-31 g/L
B)8.2 × 10-10 g/L
C)2.8 × 10-7 g/L
D)5.3 × 10-15 g/L
E)1.8 × 10-31 g/L
A)3.6 × 10-31 g/L
B)8.2 × 10-10 g/L
C)2.8 × 10-7 g/L
D)5.3 × 10-15 g/L
E)1.8 × 10-31 g/L

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
27
Rank the following salts in order of increasing molar solubility.
Salt
Ksp
BaSO4
1)1 × 10-10
AgCl
1)8 × 10-10
BaCO3
9)1 × 10-9
CdS
8 × 10-27
PbSO4
1)8 × 10-8
A)CdS < AgCl < BaSO4 < BaCO3 < PbSO4
B)CdS < AgCl < BaCO3 < BaSO4 < PbSO4
C)CdS < BaSO4 < AgCl < BaCO3 < PbSO4
D)PbSO4 < BaCO3 < AgCl < BaSO4 < CdS
E)PbSO4 < BaCO3 < BaSO4 < AgCl < CdS
Salt
Ksp
BaSO4
1)1 × 10-10
AgCl
1)8 × 10-10
BaCO3
9)1 × 10-9
CdS
8 × 10-27
PbSO4
1)8 × 10-8
A)CdS < AgCl < BaSO4 < BaCO3 < PbSO4
B)CdS < AgCl < BaCO3 < BaSO4 < PbSO4
C)CdS < BaSO4 < AgCl < BaCO3 < PbSO4
D)PbSO4 < BaCO3 < AgCl < BaSO4 < CdS
E)PbSO4 < BaCO3 < BaSO4 < AgCl < CdS

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
28
What is the molar solubility of aluminum hydroxide at 25°C? The solubility product constant for aluminum hydroxide is 4.6 × 10-33 at 25°C.
A)2.3 × 10-33 M
B)6.8 × 10-17 M
C)4.6 × 10-33 M
D)3.6 × 10-9 M
E)1.0 × 10-11 M
A)2.3 × 10-33 M
B)6.8 × 10-17 M
C)4.6 × 10-33 M
D)3.6 × 10-9 M
E)1.0 × 10-11 M

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following salts has the highest molar solubility in water?
A)CaCO3 (Ksp = 3.8 × 10-9)
B)Ni(OH)2 (Ksp = 2.0 × 10-15)
C)Fe(OH)2 (Ksp = 8 × 10-16)
D)AgBr (Ksp = 5.0 × 10-13)
E)PbI2 (Ksp = 6.5 × 10-9)
A)CaCO3 (Ksp = 3.8 × 10-9)
B)Ni(OH)2 (Ksp = 2.0 × 10-15)
C)Fe(OH)2 (Ksp = 8 × 10-16)
D)AgBr (Ksp = 5.0 × 10-13)
E)PbI2 (Ksp = 6.5 × 10-9)

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
30
What is the solubility (in g/L)of silver(I)chloride at 25°C? The solubility product constant for silver(I)chloride is 1.8 × 10-10 at 25°C.
A)5.1 × 10-2 g/L
B)2.6 × 10-8 g/L
C)1.9 × 10-3 g/L
D)1.3 × 10-8 g/L
E)2.6 × 10-1 g/L
A)5.1 × 10-2 g/L
B)2.6 × 10-8 g/L
C)1.9 × 10-3 g/L
D)1.3 × 10-8 g/L
E)2.6 × 10-1 g/L

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
31
What is the solubility (in g/L)of calcium fluoride at 25°C? The solubility product constant for calcium fluoride is 3.4 × 10-11 at 25°C.
A)0.00045 g/L
B)2.7 × 10-9 g/L
C)0.015 g/L
D)1.3 × 10-9 g/L
E)0.094 g/L
A)0.00045 g/L
B)2.7 × 10-9 g/L
C)0.015 g/L
D)1.3 × 10-9 g/L
E)0.094 g/L

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
32
Which salt has the highest molar solubility in pure water?
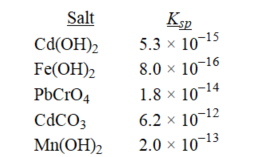
A)CdCO3
B)Cd(OH)2
C)Mn(OH)2
D)PbCrO4
E)Fe(OH)2

A)CdCO3
B)Cd(OH)2
C)Mn(OH)2
D)PbCrO4
E)Fe(OH)2

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
33
What is the molar solubility of silver(I)chloride at 25°C? The solubility product constant for silver(I)chloride is 1.8 × 10-10 at 25°C.
A)1.3 × 10-5 M
B)9.0 × 10-11 M
C)1.8 × 10-3 M
D)3.6 × 10-4 M
E)1.8 × 10-10 M
A)1.3 × 10-5 M
B)9.0 × 10-11 M
C)1.8 × 10-3 M
D)3.6 × 10-4 M
E)1.8 × 10-10 M

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
34
The silver-ion concentration in a saturated solution of silver(I)sulfate is 2.9 × 10-2 M.What is Ksp for silver(I)sulfate?
A)6.9 × 10-7
B)2.1 × 10-4
C)9.6 × 10-5
D)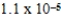
E)8.3 × 10-4
A)6.9 × 10-7
B)2.1 × 10-4
C)9.6 × 10-5
D)
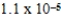
E)8.3 × 10-4

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following salts has the lowest molar solubility in water?
A)Ni(OH)2 (Ksp = 2.0 × 10-15)
B)Fe(OH)2 (Ksp = 8 × 10-16)
C)PbI2 (Ksp = 6.5 × 10-9)
D)CaCO3 (Ksp = 3.8 × 10-9)
E)AgBr (Ksp = 5.0 × 10-13)
A)Ni(OH)2 (Ksp = 2.0 × 10-15)
B)Fe(OH)2 (Ksp = 8 × 10-16)
C)PbI2 (Ksp = 6.5 × 10-9)
D)CaCO3 (Ksp = 3.8 × 10-9)
E)AgBr (Ksp = 5.0 × 10-13)

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following salts has the highest molar solubility in water?
A)SrCO3 (Ksp = 9.3 × 10-10)
B)BaSO4 (Ksp = 1.1 × 10-10 )
C)PbS (Ksp = 2.5 × 10-27)
D)BaCrO4 (Ksp = 1.2 × 10-10)
E)AgCl (Ksp = 1.8 × 10-10 )
A)SrCO3 (Ksp = 9.3 × 10-10)
B)BaSO4 (Ksp = 1.1 × 10-10 )
C)PbS (Ksp = 2.5 × 10-27)
D)BaCrO4 (Ksp = 1.2 × 10-10)
E)AgCl (Ksp = 1.8 × 10-10 )

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
37
Rank the following metal sulfides in order of increasing molar solubility in water.
Salt
Ksp
CoS
4 × 10-21
CuS
6 × 10-36
FeS
6 × 10-18
HgS
1)6 × 10-52
MnS
2)5 × 10-10
A)MnS < FeS < CoS < CuS < HgS
B)FeS < HgS < CoS < CuS < MnS
C)HgS < CuS < CoS < FeS < MnS
D)CuS < CoS < FeS < MnS < HgS
E)CoS < CuS < FeS < HgS < MnS
Salt
Ksp
CoS
4 × 10-21
CuS
6 × 10-36
FeS
6 × 10-18
HgS
1)6 × 10-52
MnS
2)5 × 10-10
A)MnS < FeS < CoS < CuS < HgS
B)FeS < HgS < CoS < CuS < MnS
C)HgS < CuS < CoS < FeS < MnS
D)CuS < CoS < FeS < MnS < HgS
E)CoS < CuS < FeS < HgS < MnS

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
38
What is the molar solubility of lead(II)chloride at 25°C? The solubility product constant for lead(II)chloride is 1.6 × 10-5 at 25°C.
A)1.6 × 10-2 M
B)1.6 × 10-5 M
C)8.0 × 10-6 M
D)4.0 × 10-3 M
E)3.2 × 10-2 M
A)1.6 × 10-2 M
B)1.6 × 10-5 M
C)8.0 × 10-6 M
D)4.0 × 10-3 M
E)3.2 × 10-2 M

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
39
The hydroxide ion concentration of a saturated solution of Cu(OH)2 is 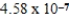 M.What is the solubility product constant for Cu(OH)2?
M.What is the solubility product constant for Cu(OH)2?
A)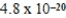
B)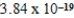
C)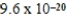
D)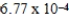
E)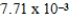
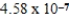 M.What is the solubility product constant for Cu(OH)2?
M.What is the solubility product constant for Cu(OH)2?A)
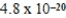
B)
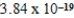
C)
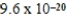
D)
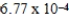
E)
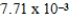

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
40
A saturated solution of which of the following salts will have the greatest molar concentration of silver ion?
A)Ag2S (Ksp = 8 × 10-51)
B)AgCl (Ksp = 1.8 × 10-10)
C)Ag2CrO4 (Ksp = 1.2 × 10-12)
D)Ag2CO3 (Ksp = 8.1 × 10-12)
E)Ag4Fe(CN)6 (Ksp = 8.5 × 10-45)
A)Ag2S (Ksp = 8 × 10-51)
B)AgCl (Ksp = 1.8 × 10-10)
C)Ag2CrO4 (Ksp = 1.2 × 10-12)
D)Ag2CO3 (Ksp = 8.1 × 10-12)
E)Ag4Fe(CN)6 (Ksp = 8.5 × 10-45)

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
41
How many moles of CaF2 will dissolve in 3.0 L of 0.041 M NaF solution? (Ksp for CaF2 = 4.0 × 10-11)
A)3.3 × 10-10
B)2.4 × 10-8
C)7.1 × 10-8
D)7.9 × 10-9
E)none of these
A)3.3 × 10-10
B)2.4 × 10-8
C)7.1 × 10-8
D)7.9 × 10-9
E)none of these

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
42
What is the pH of a saturated solution of Zn(OH)2? For Zn(OH)2,Ksp = 2.1 × 10-16.
A)5.13
B)8.57
C)5.43
D)8.87
E)7.00
A)5.13
B)8.57
C)5.43
D)8.87
E)7.00

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following will apply to a saturated solution of an ionic compound?
A)Qc < Ksp
B)Qc > Ksp
C)Qc = Ksp
D)Ksp = 1
E)Qc = 1
A)Qc < Ksp
B)Qc > Ksp
C)Qc = Ksp
D)Ksp = 1
E)Qc = 1

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
44
For which of the following will precipitation be expected?
A)Qc < Ksp
B)Qc = 1
C)Qc = Ksp
D)Qc > Ksp
E)Ksp = 1
A)Qc < Ksp
B)Qc = 1
C)Qc = Ksp
D)Qc > Ksp
E)Ksp = 1

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
45
What is the concentration of silver(I)ion in a saturated solution of silver(I)carbonate containing 0.0046 M Na2CO3? For Ag2CO3,Ksp = 8.6 × 10-12.
A)6.0 × 10-4 M
B)2.0 × 10-9 M
C)8.0 × 10-9 M
D)4.3 × 10-5 M
E)8.0 × 10-4 M
A)6.0 × 10-4 M
B)2.0 × 10-9 M
C)8.0 × 10-9 M
D)4.3 × 10-5 M
E)8.0 × 10-4 M

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
46
To 1.0 L of water,1.5 × 10-6 mol of Pb(NO3)2,6.5 × 10-6 mol of K2CrO4,and 1.0 mol of NaCl are added.What will happen?
Salt
Ksp
PbCrO4
1)8 × 10-14
PbCl2
1)6 × 10-5
A)A precipitate of KCl will form.
B)A precipitate of PbCrO4 will form.
C)A precipitate of PbCl2 will form.
D)No precipitate will form.
E)Both a precipitate of PbCl2 and a precipitate of PbCrO4 will form.
Salt
Ksp
PbCrO4
1)8 × 10-14
PbCl2
1)6 × 10-5
A)A precipitate of KCl will form.
B)A precipitate of PbCrO4 will form.
C)A precipitate of PbCl2 will form.
D)No precipitate will form.
E)Both a precipitate of PbCl2 and a precipitate of PbCrO4 will form.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
47
Rank the following salts in order of increasing molar solubility.
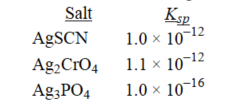
A)AgSCN < Ag2CrO4 < Ag3PO4
B)AgSCN < Ag3PO4 < Ag2CrO4
C)Ag3PO4 < Ag2CrO4 < AgSCN
D)Ag3PO4 < AgSCN < Ag2CrO4
E)Ag2CrO4 < AgSCN < Ag3PO4

A)AgSCN < Ag2CrO4 < Ag3PO4
B)AgSCN < Ag3PO4 < Ag2CrO4
C)Ag3PO4 < Ag2CrO4 < AgSCN
D)Ag3PO4 < AgSCN < Ag2CrO4
E)Ag2CrO4 < AgSCN < Ag3PO4

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
48
The solubility of La(IO3)3 in a 0.71 M KIO3 solution is 1.0 ×10-7 mol/L.Calculate Ksp for La(IO3)3.
A)7.1 × 10-8
B)3.6 × 10-22
C)3.6 × 10-1
D)3.6 × 10-8
E)none of these
A)7.1 × 10-8
B)3.6 × 10-22
C)3.6 × 10-1
D)3.6 × 10-8
E)none of these

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
49
The insoluble salts AV,B2W,C2X3,DY2,and EZ3,which were formed from the metal ions A+,B+,C3+,D2+,and E3+ and the nonmetals V1-,W2-,X2-,Y1-,and Z1-,all have the same Ksp value.Which salt has the highest molar solubility?
A)AV
B)EZ3
C)DY2
D)B2W
E)C2X3
A)AV
B)EZ3
C)DY2
D)B2W
E)C2X3

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
50
Ksp for PbF2 is 4.0 ×10-8.If a 0.032 M NaF solution is saturated with PbF2,what is [Pb2+] in solution?
A)4.1 × 10-11 M
B)1.3 × 10-9 M
C)1.3 × 10-6 M
D)1.2 × 10-3 M
E)3.9 × 10-5 M
A)4.1 × 10-11 M
B)1.3 × 10-9 M
C)1.3 × 10-6 M
D)1.2 × 10-3 M
E)3.9 × 10-5 M

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
51
What is the hydroxide-ion concentration of a saturated solution of Ni(OH)2? For Ni(OH)2,Ksp = 2.0 × 10-15.
A)2.8 × 10-3 M
B)7.9 × 10-6 M
C)1.0 × 10-7 M
D)2.7 × 10-2 M
E)1.6 × 10-5 M
A)2.8 × 10-3 M
B)7.9 × 10-6 M
C)1.0 × 10-7 M
D)2.7 × 10-2 M
E)1.6 × 10-5 M

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
52
Suppose 50.00 mL of 2.0 × 10-6 M Fe(NO3)3 is added to 50.00 mL of 2.0 ×10-4 M KIO3.Which of the following statements is true? For Fe(IO3)3,Ksp = 1.0 × 10-14.
A)A precipitate forms because Qc > Ksp.
B)A precipitate forms because Qc < Ksp.
C)No precipitate forms because Qc < Ksp.
D)No precipitate forms because Qc = Ksp.
E)No precipitate forms because Qc > Ksp.
A)A precipitate forms because Qc > Ksp.
B)A precipitate forms because Qc < Ksp.
C)No precipitate forms because Qc < Ksp.
D)No precipitate forms because Qc = Ksp.
E)No precipitate forms because Qc > Ksp.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
53
What is the molar solubility of MgF2 in a 0.40 M NaF solution? For MgF2,Ksp = 8.4 × 10-8.
A)1.0 × 10-7 M
B)1.4 × 10-4 M
C)2.1 × 10-7 M
D)7.1 × 10-4 M
E)5.3 × 10-7 M
A)1.0 × 10-7 M
B)1.4 × 10-4 M
C)2.1 × 10-7 M
D)7.1 × 10-4 M
E)5.3 × 10-7 M

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
54
Which of Figures I-IV represent(s)the result of mixing aqueous solutions of Na2S and NiCl2 in which the ion product Qc > Ksp for the insoluble product? (C = cation,A = anion) 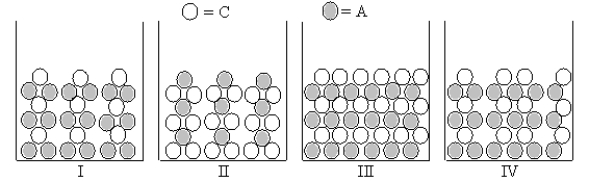
A)both I and II
B)only I
C)only II
D)only III
E)only IV

A)both I and II
B)only I
C)only II
D)only III
E)only IV

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
55
Which Figures I-IV represent(s)the result of mixing aqueous solutions of NaOH and CuCl2 in which the ion product Qc > Ksp for the insoluble product? (C = cation,A = anion) 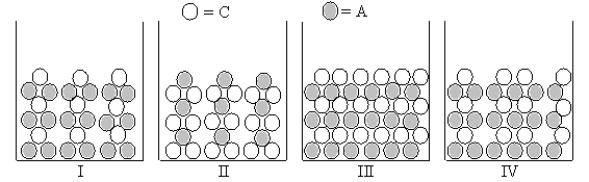
A)only II
B)both I and II
C)only IV
D)only I
E)only III

A)only II
B)both I and II
C)only IV
D)only I
E)only III

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
56
The figure below represents the result of adding which of the following aqueous solutions to a filtered,saturated solution of AgCl? 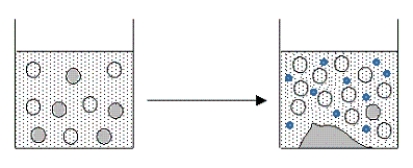
A)only NaCl(aq)
B)only HNO3(aq)
C)HCl(aq)or NaCl(aq)
D)only HCl(aq)
E)HCl(aq)or HNO3(aq)

A)only NaCl(aq)
B)only HNO3(aq)
C)HCl(aq)or NaCl(aq)
D)only HCl(aq)
E)HCl(aq)or HNO3(aq)

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
57
In which of these solutions would silver(I)carbonate have the lowest molar solubility? For silver(I)carbonate,Ksp = 8.5 × 10-12.
A)0.03 M H2CO3
B)0.1 M AgNO3
C)0.01 M AgNO3
D)0.1 M Na2CO3
E)pure water
A)0.03 M H2CO3
B)0.1 M AgNO3
C)0.01 M AgNO3
D)0.1 M Na2CO3
E)pure water

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
58
Which salt has the lowest molar solubility in pure water?

A)PbCrO4
B)Fe(OH)2
C)CdCO3
D)Cd(OH)2
E)Mn(OH)2

A)PbCrO4
B)Fe(OH)2
C)CdCO3
D)Cd(OH)2
E)Mn(OH)2

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
59
In which of the following solutions would silver(I)phosphate,Ag3PO4,be least soluble?
A)0.10 M Na3PO4
B)0.10 M AgNO3
C)0.10 M Na2HPO4
D)0.10 M HNO3
E)0.10 M NaH2PO4
A)0.10 M Na3PO4
B)0.10 M AgNO3
C)0.10 M Na2HPO4
D)0.10 M HNO3
E)0.10 M NaH2PO4

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
60
What is the molar solubility of MgF2 in a 0.36 M Mg(NO3)2 solution? For MgF2,Ksp = 8.4 × 10-8.
A)8.0 × 10-8 M
B)2.4 × 10-4 M
C)2.0 × 10-8 M
D)4.8 × 10-4 M
E)3.2 × 10-3 M
A)8.0 × 10-8 M
B)2.4 × 10-4 M
C)2.0 × 10-8 M
D)4.8 × 10-4 M
E)3.2 × 10-3 M

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
61
A 5.0 × 10-4 M solution of MnSO4 is gradually made more basic by adding NaOH.At what pH will manganese(II)hydroxide begin to precipitate? For Mn(OH)2,Ksp = 2.0 × 10-13.
A)4.60
B)9.57
C)4.70
D)9.30
E)9.40
A)4.60
B)9.57
C)4.70
D)9.30
E)9.40

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
62
For which pair of cations would the addition of dilute hydrobromic acid precipitate one but not the other?
A)Ag+ and Ca2+
B)Hg22+ and Ag+
C)Ba2+ and Na+
D)Ca2+ and Ba2+
E)Pb2+ and Ag+
A)Ag+ and Ca2+
B)Hg22+ and Ag+
C)Ba2+ and Na+
D)Ca2+ and Ba2+
E)Pb2+ and Ag+

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
63
What is the maximum concentration of carbonate ions that will precipitate BaCO3 but not MgCO3 from a solution that is 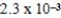 M each in Mg2+ and Ba2+? For MgCO3,Ksp = 1.0 × 10-5 and for BaCO3,Ksp = 2.6 × 10-9.
M each in Mg2+ and Ba2+? For MgCO3,Ksp = 1.0 × 10-5 and for BaCO3,Ksp = 2.6 × 10-9.
A)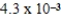 M
M
B)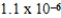 M
M
C)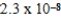 M
M
D)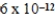 M
M
E)2.6 × 10-14 M
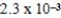 M each in Mg2+ and Ba2+? For MgCO3,Ksp = 1.0 × 10-5 and for BaCO3,Ksp = 2.6 × 10-9.
M each in Mg2+ and Ba2+? For MgCO3,Ksp = 1.0 × 10-5 and for BaCO3,Ksp = 2.6 × 10-9.A)
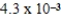 M
MB)
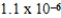 M
MC)
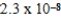 M
MD)
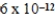 M
ME)2.6 × 10-14 M

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
64
If 275 mL of 1 × 10-7 M AgNO3 is mixed with 275 mL of 1 × 10-8 M NaI,what will occur? For AgI,Ksp = 8.3 × 10-17.
A)Sodium nitrate will precipitate.
B)Silver(I)nitrate will precipitate.
C)Sodium iodide will precipitate.
D)Silver(I)iodide will precipitate.
E)No precipitate will form.
A)Sodium nitrate will precipitate.
B)Silver(I)nitrate will precipitate.
C)Sodium iodide will precipitate.
D)Silver(I)iodide will precipitate.
E)No precipitate will form.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
65
What is the maximum hydroxide-ion concentration that a 0.025 M MgCl2 solution could have without causing the precipitation of Mg(OH)2? For Mg(OH)2,Ksp = 1.8 × 10-11.
A)4.2 × 10-6
B)1.7 × 10-4
C)1.2 × 10-8
D)7.2 × 10-9
E)2.7 × 10-5
A)4.2 × 10-6
B)1.7 × 10-4
C)1.2 × 10-8
D)7.2 × 10-9
E)2.7 × 10-5

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
66
If 370 mL of 1 × 10-8 M Al(NO3)3 is mixed with 370 mL of 1 × 10-8 M NaOH,what will occur? For Al(OH)3,Ksp = 4.6 × 10-33.
A)Aluminum hydroxide will precipitate.
B)Sodium hydroxide will precipitate.
C)Aluminum nitrate will precipitate.
D)Sodium nitrate will precipitate.
E)No precipitate will form.
A)Aluminum hydroxide will precipitate.
B)Sodium hydroxide will precipitate.
C)Aluminum nitrate will precipitate.
D)Sodium nitrate will precipitate.
E)No precipitate will form.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
67
What is the minimum mass of Na2CO3 that must be added to 75.9 mL of a 9.0 × 10-4 M AgNO3 solution in order for precipitation to occur? For Ag2CO3,Ksp = 8.6 × 10-12 .
A)7.2 × 10-3 g
B)3.1 × 10-4 g
C)3.6 × 10-3 g
D)7.7 × 10-8 g
E)8.5 × 10-5 g
A)7.2 × 10-3 g
B)3.1 × 10-4 g
C)3.6 × 10-3 g
D)7.7 × 10-8 g
E)8.5 × 10-5 g

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
68
Suppose 50.00 mL of a 1 × 10-7 M solution of lead(II)nitrate is mixed with 50.00 mL of a 1 × 10-8 solution of sodium phosphate.Which of the following statements is true? For lead(II)phosphate,Ksp = 1 × 10-44.
A)A precipitate forms because Qc < Ksp.
B)No precipitate forms because Qc > Ksp.
C)A precipitate forms because Qc > Ksp.
D)No precipitate forms because Qc = Ksp.
E)No precipitate forms because Qc < Ksp.
A)A precipitate forms because Qc < Ksp.
B)No precipitate forms because Qc > Ksp.
C)A precipitate forms because Qc > Ksp.
D)No precipitate forms because Qc = Ksp.
E)No precipitate forms because Qc < Ksp.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
69
What is the maximum volume of 8.6 × 10-5 M K2CrO4 that,added to 40.4 mL of a solution that is 5.1 × 10-5 M Ba(NO3)2 and 1.5 × 10-6 M Pb(NO3)2,will precipitate PbCrO4 but not BaCrO4? For PbCrO4,Ksp = 1.8 × 10-14,and for BaCrO4,Ksp = 1.2 × 10-10.
A)40 mL
B)1.1 mL
C)1.0 mL
D)0.0056 mL
E)37 mL
A)40 mL
B)1.1 mL
C)1.0 mL
D)0.0056 mL
E)37 mL

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
70
A solution contains 0.018 mol each of I-,Br-,and Cl-.When the solution is mixed with 200 mL of 0.24 M AgNO3,how much AgCl(s)precipitates out?
Ksp
AgI
= 1)5 × 10-16
Ksp
AgBr
= 5)0 × 10-13
Ksp
AgCl
= 1)6 × 10-10
A)5.0 g
B)3.3 g
C)2.6 g
D)0.0 g
E)1.7 g
Ksp
AgI
= 1)5 × 10-16
Ksp
AgBr
= 5)0 × 10-13
Ksp
AgCl
= 1)6 × 10-10
A)5.0 g
B)3.3 g
C)2.6 g
D)0.0 g
E)1.7 g

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
71
If 430 mL of 1 × 10-4 M Ca(NO3)2 is mixed with 430 mL of 1 × 10-4 M NaF,what will occur? For CaF2,Ksp = 3.4 × 10-11.
A)No precipitate will form.
B)Sodium nitrate will precipitate.
C)Calcium nitrate will precipitate.
D)Calcium fluoride will precipitate.
E)Sodium fluoride will precipitate.
A)No precipitate will form.
B)Sodium nitrate will precipitate.
C)Calcium nitrate will precipitate.
D)Calcium fluoride will precipitate.
E)Sodium fluoride will precipitate.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
72
Silver nitrate (AgNO3)is slowly added to a solution containing 0.100 M Br− and 0.050 M FeCN64− until a precipitate just forms.What is the molar concentration of Ag+ just as the precipitate forms? AgBr Ksp = 5.0 × 10-13 and Ag4FeCN6 Ksp = 8.5 × 10-45.
A)2.0 × 10-11 M Ag+
B)5.0 × 10-12 M Ag+
C)1.0 × 10-11 M Ag+
D)3.3 × 10-12 M Ag+
E)1.7 × 10-43 M Ag+
A)2.0 × 10-11 M Ag+
B)5.0 × 10-12 M Ag+
C)1.0 × 10-11 M Ag+
D)3.3 × 10-12 M Ag+
E)1.7 × 10-43 M Ag+

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
73
A solution is 0.010 M in each of Pb(NO3)2,Mn(NO3)2,and Zn(NO3)2.Solid NaOH is added until the pH of the solution is 8.50.Which of the following statements is true?
Salt
Ksp
Pb(OH)2
1)4 × 10-20
Mn(OH)2
2)0 × 10-13
Zn(OH)2
2)1 × 10-16
A)Only Mn(OH)2 will precipitate.
B)All three hydroxides will precipitate.
C)Only Pb(OH)2 will precipitate.
D)No precipitate will form.
E)Only Zn(OH)2 and Pb(OH)2 will precipitate.
Salt
Ksp
Pb(OH)2
1)4 × 10-20
Mn(OH)2
2)0 × 10-13
Zn(OH)2
2)1 × 10-16
A)Only Mn(OH)2 will precipitate.
B)All three hydroxides will precipitate.
C)Only Pb(OH)2 will precipitate.
D)No precipitate will form.
E)Only Zn(OH)2 and Pb(OH)2 will precipitate.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
74
If 500 mL of 1.3 × 10-6 M AgNO3 is mixed with 500 mL of 1.3 × 10-6 M NaBr,what will occur? For AgBr,Ksp = 5 × 10-13.
A)Silver(I)bromide will precipitate.
B)The concentration of Ag+ will be 1.3 × 10-6 M.
C)6.5 × 10-7 mol of AgBr will form.
D)No precipitation will occur.
E)Sodium bromide will precipitate.
A)Silver(I)bromide will precipitate.
B)The concentration of Ag+ will be 1.3 × 10-6 M.
C)6.5 × 10-7 mol of AgBr will form.
D)No precipitation will occur.
E)Sodium bromide will precipitate.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
75
Solid KCN is added to a solution composed of 0.10 M Ag+ and 0.10 M Zn2+ just until a precipitate forms.What is the composition of this initial precipitate? AgCN Ksp = 2.2 × 10-16 and Zn(CN)2 Ksp = 3 × 10-16.
A)The precipitate is pure AgCN(s).
B)The precipitateis pure Zn(CN)2(s).
C)The precipitate is a mixture of AgCN(s)and Zn(CN)2(s).
D)The precipitate is a mixture of KCN(s)and AgCN(s).
E)The precipitate is a mixture of KCN(s)and Zn(CN)2(s).
A)The precipitate is pure AgCN(s).
B)The precipitateis pure Zn(CN)2(s).
C)The precipitate is a mixture of AgCN(s)and Zn(CN)2(s).
D)The precipitate is a mixture of KCN(s)and AgCN(s).
E)The precipitate is a mixture of KCN(s)and Zn(CN)2(s).

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
76
What is the minimum concentration of Pb2+ required to begin precipitating Pb(OH)2(s)in a solution of pH 10.78? For Pb(OH)2,Ksp = 1.4 × 10-20.
A)3.0 × 10-4 M
B)8.4 × 10-10 M
C)2.3 × 10-17 M
D)3.9 × 10-14 M
E)1.2 × 10-22 M
A)3.0 × 10-4 M
B)8.4 × 10-10 M
C)2.3 × 10-17 M
D)3.9 × 10-14 M
E)1.2 × 10-22 M

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
77
What will happen if 0.1 mol of solid silver(I)nitrate is added to 1.0 L of a saturated solution of silver(I)chromate? For Ag2CrO4,Ksp = 2.4 × 10-12.
A)The AgNO3 will settle to the bottom without dissolving.
B)The concentration of CrO42- will increase.
C)Some Ag2CrO4 will precipitate.
D)Nothing will happen.
E)The concentration of Ag+ in solution will not change.
A)The AgNO3 will settle to the bottom without dissolving.
B)The concentration of CrO42- will increase.
C)Some Ag2CrO4 will precipitate.
D)Nothing will happen.
E)The concentration of Ag+ in solution will not change.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
78
Sodium chloride is added slowly to a solution that is 0.010 M in Cu+,Ag+,and Au+.The Ksp values for the chloride salts are 1.9 × 10-7,1.6 × 10-10,and 2.0 × 10-13,respectively.Which compound will precipitate first?
A)AuCl(s)
B)All will precipitate at the same time.
C)It cannot be determined.
D)AgCl(s)
E)CuCl(s)
A)AuCl(s)
B)All will precipitate at the same time.
C)It cannot be determined.
D)AgCl(s)
E)CuCl(s)

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
79
What is the maximum Sr2+ concentration possible in a solution that has a 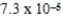 M sulfide-ion concentration without precipitating strontium sulfate? For SrSO4,Ksp = 2.5 × 10-7.
M sulfide-ion concentration without precipitating strontium sulfate? For SrSO4,Ksp = 2.5 × 10-7.
A)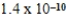 M
M
B)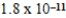 M
M
C)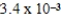 M
M
D)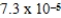 M
M
E)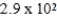 M
M
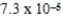 M sulfide-ion concentration without precipitating strontium sulfate? For SrSO4,Ksp = 2.5 × 10-7.
M sulfide-ion concentration without precipitating strontium sulfate? For SrSO4,Ksp = 2.5 × 10-7.A)
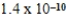 M
MB)
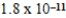 M
MC)
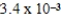 M
MD)
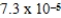 M
ME)
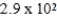 M
M
Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following solutions should be added to a solution containing both copper(II)ions and silver(I)ions in order to precipitate only one of the ions?
A)HCl(aq)
B)H2S(aq)
C)HNO3(aq)
D)H2S(aq)+ HCl(aq)
E)H2S(aq)+ HNO3(aq)
A)HCl(aq)
B)H2S(aq)
C)HNO3(aq)
D)H2S(aq)+ HCl(aq)
E)H2S(aq)+ HNO3(aq)

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck


