Deck 20: Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/90
Play
Full screen (f)
Deck 20: Nuclear Chemistry
1
Which particle has the same mass as a beta particle?
A)a neutron
B)a proton
C)a gamma ray
D)an alpha particle
E)a positron
A)a neutron
B)a proton
C)a gamma ray
D)an alpha particle
E)a positron
a positron
2
When the radioactive nuclide 110In undergoes positron emission,what is the product nuclide?
A)(110Sn)
B)(110Cd)
C)(109Sn)
D)(109In)
E)(109Sb)
A)(110Sn)
B)(110Cd)
C)(109Sn)
D)(109In)
E)(109Sb)
(110Cd)
3
A sodium nucleus, 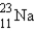 ,is bombarded with alpha particles to form a radioactive nuclide.This product nuclide can decay by several routes.Which of the following sets of products does not represent a potential route of decay of the product nuclide?
,is bombarded with alpha particles to form a radioactive nuclide.This product nuclide can decay by several routes.Which of the following sets of products does not represent a potential route of decay of the product nuclide?
A)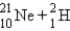
B)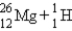
C)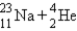
D)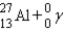
E)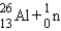
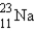 ,is bombarded with alpha particles to form a radioactive nuclide.This product nuclide can decay by several routes.Which of the following sets of products does not represent a potential route of decay of the product nuclide?
,is bombarded with alpha particles to form a radioactive nuclide.This product nuclide can decay by several routes.Which of the following sets of products does not represent a potential route of decay of the product nuclide?A)
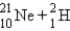
B)
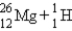
C)
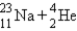
D)
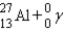
E)
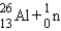
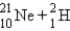
4
When 103Pd undergoes electron capture,what is the product nuclide?
A)(103Ag)
B)(101Rh)
C)(107Ag)
D)(103Rh)
E)(104Cd)
A)(103Ag)
B)(101Rh)
C)(107Ag)
D)(103Rh)
E)(104Cd)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements about 248Bk is incorrect?
A)If 248Bk were to undergo spontaneous fission,the products would be 247Bk and a neutron.
B)If 248Bk were to undergo beta decay,the products would be 248Cf and a beta particle.
C)If 248Bk were to undergo alpha decay,the products would be 244Am and an alpha particle.
D)If 248Bk were to undergo electron capture,the only product would be 248Cm.
E)If a metastable form of 248Bk were to undergo gamma decay,the products would be 248Bk and a gamma ray.
A)If 248Bk were to undergo spontaneous fission,the products would be 247Bk and a neutron.
B)If 248Bk were to undergo beta decay,the products would be 248Cf and a beta particle.
C)If 248Bk were to undergo alpha decay,the products would be 244Am and an alpha particle.
D)If 248Bk were to undergo electron capture,the only product would be 248Cm.
E)If a metastable form of 248Bk were to undergo gamma decay,the products would be 248Bk and a gamma ray.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following nuclides will produce 233Pa upon undergoing alpha decay?
A)(234Pa)
B)(233U)
C)(229Ra)
D)(237Np)
E)(233Th)
A)(234Pa)
B)(233U)
C)(229Ra)
D)(237Np)
E)(233Th)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
7
When the radioactive nuclide 62Zn undergoes electron capture,what is the product nuclide?
A)(61Cu)
B)(61Ga)
C)(62Cu)
D)(61Zn)
E)(62Ga)
A)(61Cu)
B)(61Ga)
C)(62Cu)
D)(61Zn)
E)(62Ga)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
8
The gamma ray emission from the decay of cobalt-60 is used in cancer therapies.Cobalt-60 decays by the emission of two gamma rays followed by beta emission.What is the final product of this decay process?
A)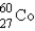
B)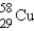
C)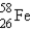
D)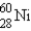
E)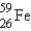
A)
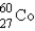
B)
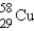
C)
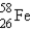
D)
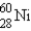
E)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
9
Which particle does the nuclide symbol 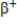 represent?
represent?
A)positron
B)helium nucleus
C)electron
D)proton
E)gamma photon
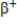 represent?
represent?A)positron
B)helium nucleus
C)electron
D)proton
E)gamma photon

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
10
The radioactive nuclide,22Na,undergoes decay by emitting a positron.What is the nuclear composition of the product nuclide?
A)12 protons and 10 neutrons
B)11 protons and 11 neutrons
C)11 protons and 10 neutrons
D)9 protons and 12 neutrons
E)10 protons and 12 neutrons
A)12 protons and 10 neutrons
B)11 protons and 11 neutrons
C)11 protons and 10 neutrons
D)9 protons and 12 neutrons
E)10 protons and 12 neutrons

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following nuclides is most likely to be radioactive?
A)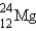
B)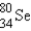
C)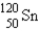
D)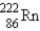
E)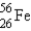
A)
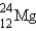
B)
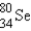
C)
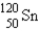
D)
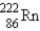
E)
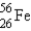

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following nuclides will produce 192Pt upon undergoing beta decay?
A)(192Ir)
B)(193Pt)
C)(192Au)
D)(196Hg)
E)(188Os)
A)(192Ir)
B)(193Pt)
C)(192Au)
D)(196Hg)
E)(188Os)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
13
When the radioactive nuclide 198Po undergoes alpha emission,what is the product nuclide?
A)(196Pb)
B)(202Rn)
C)(196At)
D)(194Pb)
E)(198Rn)
A)(196Pb)
B)(202Rn)
C)(196At)
D)(194Pb)
E)(198Rn)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
14
When 25Na undergoes beta emission,what is the product nuclide?
A)(25Ne)
B)(24Mg)
C)(24Na)
D)(24Ne)
E)(25Mg)
A)(25Ne)
B)(24Mg)
C)(24Na)
D)(24Ne)
E)(25Mg)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
15
In the ejection of a beta particle from the nucleus,which of the following occurs?
A)A neutron is converted to a proton and an electron.
B)A neutron is converted to an electron and an alpha particle.
C)A positron is converted to a neutron and a proton.
D)A neutron is converted to a positron,an electron,and a gamma ray.
E)A proton is converted to a neutron and a positron.
A)A neutron is converted to a proton and an electron.
B)A neutron is converted to an electron and an alpha particle.
C)A positron is converted to a neutron and a proton.
D)A neutron is converted to a positron,an electron,and a gamma ray.
E)A proton is converted to a neutron and a positron.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following nuclides will produce 243Am upon undergoing alpha decay?
A)(247Bk)
B)(239Np)
C)(243Pu)
D)(243Cm)
E)(244Am)
A)(247Bk)
B)(239Np)
C)(243Pu)
D)(243Cm)
E)(244Am)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following nuclides is most likely to be radioactive?
A)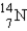
B)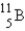
C)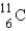
D)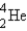
E)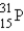
A)
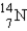
B)
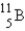
C)
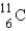
D)
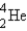
E)
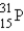

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
18
When a nucleus undergoes radioactive decay,its new mass number is
A)always less than its original mass number.
B)never more than its original mass number.
C)never less than its original mass number.
D)always the same as its original mass number.
E)always more than its original mass number.
A)always less than its original mass number.
B)never more than its original mass number.
C)never less than its original mass number.
D)always the same as its original mass number.
E)always more than its original mass number.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
19
55Mn can be prepared by electron capture from which of the following?
A)(56Mn)
B)(55Fe)
C)(55Cr)
D)(51V)
E)(57Co)
A)(56Mn)
B)(55Fe)
C)(55Cr)
D)(51V)
E)(57Co)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
20
For which of the following radioactive decay processes does the atomic number not change?
A)electron capture
B)positron emission
C)alpha emission
D)gamma emission
E)beta emission
A)electron capture
B)positron emission
C)alpha emission
D)gamma emission
E)beta emission

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is the most probable mode of radioactive decay for the radioactive nuclide 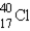 ?
?
A)beta emission
B)gamma emission
C)neutron emission
D)alpha emission
E)positron emission
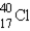 ?
?A)beta emission
B)gamma emission
C)neutron emission
D)alpha emission
E)positron emission

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
22
Which types of processes are likely when the neutron-to-proton ratio in a nucleus is too low?
I
Α decay
II
Β decay
III
Positron emission
IV
Electron capture
A)III and IV only
B)I and II only
C)II,III,and IV
D)II and IV only
E)II and III only
I
Α decay
II
Β decay
III
Positron emission
IV
Electron capture
A)III and IV only
B)I and II only
C)II,III,and IV
D)II and IV only
E)II and III only

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
23
A radioactive isotope decays by α emission followed by two β emissions.What is the change in the mass number and atomic number of the original isotope?
A)The mass number decreases by 4 and the atomic number is unchanged.
B)The mass number increases by 4 and the atomic number increases by 2.
C)The mass number increases by 4 and the atomic number increases by 4.
D)The mass number decreases by 2 and the atomic number decreases by 2.
E)none of the above
A)The mass number decreases by 4 and the atomic number is unchanged.
B)The mass number increases by 4 and the atomic number increases by 2.
C)The mass number increases by 4 and the atomic number increases by 4.
D)The mass number decreases by 2 and the atomic number decreases by 2.
E)none of the above

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
24
The following reaction is an example of what type of process? 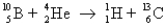
A)alpha decay
B)deprotonation
C)fission
D)transmutation
E)none of these
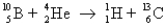
A)alpha decay
B)deprotonation
C)fission
D)transmutation
E)none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
25
Nuclides with too many neutrons to be in the band of stability are most likely to decay by what mode?
A)β-
B)fission
C)α
D)β+
E)electron capture
A)β-
B)fission
C)α
D)β+
E)electron capture

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
26
Which of these is not a transuranium element?
A)plutonium
B)neptunium
C)curium
D)thorium
E)americium
A)plutonium
B)neptunium
C)curium
D)thorium
E)americium

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
27
When the nucleus of an element undergoes beta emission
A)the mass number increases by one.
B)the mass number decreases by one.
C)the atomic number decreases by one.
D)the number of neutrons increases by one.
E)the atomic number increases by one.
A)the mass number increases by one.
B)the mass number decreases by one.
C)the atomic number decreases by one.
D)the number of neutrons increases by one.
E)the atomic number increases by one.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following particles cannot be accelerated to high speeds in a particle accelerator or cyclotron?
A)neutrons
B)electrons
C)protons
D)alpha particles
E)hydrogen nuclei
A)neutrons
B)electrons
C)protons
D)alpha particles
E)hydrogen nuclei

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
29
A particular nuclear bombardment reaction is represented by the abbreviated notation 147N(n,α).What is the identity of the nuclide X?
A)(115B)
B)(137N)
C)(84Be)
D)(156C)
E)(168O)
A)(115B)
B)(137N)
C)(84Be)
D)(156C)
E)(168O)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
30
A nucleus located to the left of the band of stability is expected to undergo what type of nuclear decay?
A)alpha emission
B)electron capture
C)positron emission
D)fission
E)electron emission
A)alpha emission
B)electron capture
C)positron emission
D)fission
E)electron emission

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
31
The nuclide 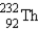 is radioactive.When one of these atoms decays,a series of α and β- particle emissions occurs,taking the atom through many transformations to end up as an atom of
is radioactive.When one of these atoms decays,a series of α and β- particle emissions occurs,taking the atom through many transformations to end up as an atom of 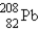
)How many α particles are emitted in converting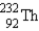
Into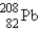
?
A)6
B)2
C)214
D)8
E)4
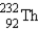 is radioactive.When one of these atoms decays,a series of α and β- particle emissions occurs,taking the atom through many transformations to end up as an atom of
is radioactive.When one of these atoms decays,a series of α and β- particle emissions occurs,taking the atom through many transformations to end up as an atom of 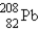
)How many α particles are emitted in converting
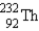
Into
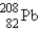
?
A)6
B)2
C)214
D)8
E)4

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
32
What is the abbreviated notation for the following nuclear bombardment reaction? 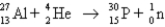
A)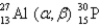
B)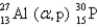
C)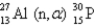
D)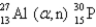
E)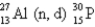
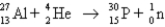
A)
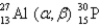
B)
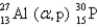
C)
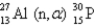
D)
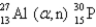
E)
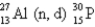

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
33
When a neutron in the nucleus is converted to a proton,which of the following is emitted?
A)positron
B)beta particle
C)alpha particle
D)deuteron
E)neutron
A)positron
B)beta particle
C)alpha particle
D)deuteron
E)neutron

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
34
The first nuclear reaction that was ever observed occurred when nitrogen-14 was bombarded with alpha particles.One product was a proton,and the other was
A)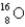 .
.
B)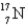 .
.
C)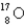 .
.
D)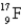 .
.
E)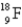 .
.
A)
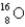 .
.B)
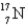 .
.C)
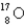 .
.D)
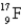 .
.E)
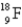 .
.
Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is the most likely mode of decay for the radioactive nuclide 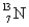 ?
?
A)positron emission
B)gamma radiation
C)beta emission
D)alpha emission
E)neutron emission
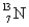 ?
?A)positron emission
B)gamma radiation
C)beta emission
D)alpha emission
E)neutron emission

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
36
In the radioactive decay series of plutonium-244 to form lead-208,how many alpha particles and how many beta particles are emitted per plutonium atom?
A)6 alpha particles and 6 beta particles
B)3 alpha particles and 3 beta particles
C)9 alpha particles and 6 beta particles
D)12 alpha particles and 8 beta particles
E)18 alpha particles and 9 beta particles
A)6 alpha particles and 6 beta particles
B)3 alpha particles and 3 beta particles
C)9 alpha particles and 6 beta particles
D)12 alpha particles and 8 beta particles
E)18 alpha particles and 9 beta particles

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
37
When 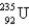 absorbs a neutron,fission occurs.One possible fission pathway is as follows:
absorbs a neutron,fission occurs.One possible fission pathway is as follows: 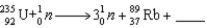
What is the missing isotope?
A)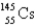
B)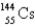
C)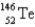
D)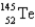
E)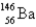
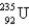 absorbs a neutron,fission occurs.One possible fission pathway is as follows:
absorbs a neutron,fission occurs.One possible fission pathway is as follows: 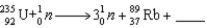
What is the missing isotope?
A)
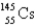
B)
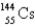
C)
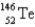
D)
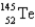
E)
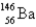

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
38
Which is the most likely mode of radioactive decay for the radioactive nuclide carbon-14?
A)gamma emission
B)alpha emission
C)electron capture
D)positron emission
E)beta emission
A)gamma emission
B)alpha emission
C)electron capture
D)positron emission
E)beta emission

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
39
Which particle has a +2 charge and is produced during the decay of some radioactive elements?
A)alpha particle
B)beta particle
C)proton
D)positron
E)deuteron
A)alpha particle
B)beta particle
C)proton
D)positron
E)deuteron

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
40
When 235U collides with one neutron,fission occurs.What is one possible set of products?
A)four neutrons,90Sr,and 140Xe
B)four neutrons,90Sr,and 139Xe
C)four neutrons,90Sr,and 139Ce
D)four neutrons,90Sr,and 141Xe
E)four neutrons,90Sr,and 142Xe
A)four neutrons,90Sr,and 140Xe
B)four neutrons,90Sr,and 139Xe
C)four neutrons,90Sr,and 139Ce
D)four neutrons,90Sr,and 141Xe
E)four neutrons,90Sr,and 142Xe

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
41
A Geiger counter measures radiation by detecting
A)cations produced from radiation colliding with phosphor gases.
B)alpha and beta particles as they strike a detector window.
C)the increase in temperature when a gas is struck by radiation.
D)flashes of light emitted from a phosphor affected by radiation.
E)electrons released when gas atoms are ionized by the radiation.
A)cations produced from radiation colliding with phosphor gases.
B)alpha and beta particles as they strike a detector window.
C)the increase in temperature when a gas is struck by radiation.
D)flashes of light emitted from a phosphor affected by radiation.
E)electrons released when gas atoms are ionized by the radiation.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
42
The activity of a radioactive source
A)is a measure of the number of nuclear disintegrations per second.
B)may be quantified in units of curies (Ci).
C)is a measure of the energy released per kilogram of tissue.
D)A and B
E)B and C
A)is a measure of the number of nuclear disintegrations per second.
B)may be quantified in units of curies (Ci).
C)is a measure of the energy released per kilogram of tissue.
D)A and B
E)B and C

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
43
What is the decay constant for a particular radioactive element that has a half-life of 5.66 years?
A)0.161/year
B)0.113/year
C)7.18 × 10-3/h
D)25.8/s
E)0.122/year
A)0.161/year
B)0.113/year
C)7.18 × 10-3/h
D)25.8/s
E)0.122/year

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
44
Use the following table to assist in answering the question below.
Nuclide
Half-Life
Uranium-238
4)51 × 109 years
Uranium-234
2)48 × 105 years
Thorium-230
8)0 × 104 years
Radium-226
1)62 × 103 years
Lead-210
20)4 years
The rate constant for the decay of unstable nuclide X by alpha-particle emission is 1.17 × 10-6 / day.What is the identity of X?
A)radium-226
B)thorium-230
C)uranium-238
D)uranium-234
E)lead-210
Nuclide
Half-Life
Uranium-238
4)51 × 109 years
Uranium-234
2)48 × 105 years
Thorium-230
8)0 × 104 years
Radium-226
1)62 × 103 years
Lead-210
20)4 years
The rate constant for the decay of unstable nuclide X by alpha-particle emission is 1.17 × 10-6 / day.What is the identity of X?
A)radium-226
B)thorium-230
C)uranium-238
D)uranium-234
E)lead-210

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
45
The half-life of 42K is 12.5 h.How much will remain after 75 h if the original sample contained 256 g of 42K?
A)22 g
B)4.0 g
C)15 g
D)20 g
E)13 g
A)22 g
B)4.0 g
C)15 g
D)20 g
E)13 g

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
46
After 3.2 hours, 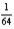 of the initial amount of a particular radioactive nuclide remains unchanged.What is the half-life of the nuclide?
of the initial amount of a particular radioactive nuclide remains unchanged.What is the half-life of the nuclide?
A)32 min
B)45 min
C)60 min
D)74 min
E)20 min
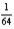 of the initial amount of a particular radioactive nuclide remains unchanged.What is the half-life of the nuclide?
of the initial amount of a particular radioactive nuclide remains unchanged.What is the half-life of the nuclide?A)32 min
B)45 min
C)60 min
D)74 min
E)20 min

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
47
What is the percent activity of a radioactive sample (relative to its original activity)that has undergone four half-lives of decay?
A)6.25%
B)25%
C)12.5%
D)3.13%
E)75.0%
A)6.25%
B)25%
C)12.5%
D)3.13%
E)75.0%

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following corresponds to the most rapid nuclear decay?
A)t1/2 = 1.0 × 103 min
B)t1/2 = 1.0 × 109 year
C)k = 1.0 × 10-3/year
D)k = 1.0 × 10-1/day
E)k = 1.0 × 10-5/s
A)t1/2 = 1.0 × 103 min
B)t1/2 = 1.0 × 109 year
C)k = 1.0 × 10-3/year
D)k = 1.0 × 10-1/day
E)k = 1.0 × 10-5/s

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
49
The half-life of the radioactive nuclide 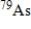 is 9.0 min.What is the activity of a 4.7-µg sample of
is 9.0 min.What is the activity of a 4.7-µg sample of 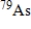
? The molar mass of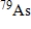
Is 78.921 g/mol.(1 Ci = 3.700 × 1010 disintegrations/s)
A)1.8×103Ci
B)1.1×105Ci
C)1.2×103Ci
D)7.5×104Ci
E)1.1×10-10Ci
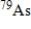 is 9.0 min.What is the activity of a 4.7-µg sample of
is 9.0 min.What is the activity of a 4.7-µg sample of 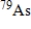
? The molar mass of
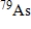
Is 78.921 g/mol.(1 Ci = 3.700 × 1010 disintegrations/s)
A)1.8×103Ci
B)1.1×105Ci
C)1.2×103Ci
D)7.5×104Ci
E)1.1×10-10Ci

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
50
A scintillation counter measures radiation by detecting
A)cations produced from radiation colliding with phosphor gases.
B)electrons released when gas atoms are ionized by the radiation.
C)alpha and beta particles as they strike a detector window.
D)the increase in temperature when a gas is struck by radiation.
E)flashes of light emitted from a phosphor affected by radiation.
A)cations produced from radiation colliding with phosphor gases.
B)electrons released when gas atoms are ionized by the radiation.
C)alpha and beta particles as they strike a detector window.
D)the increase in temperature when a gas is struck by radiation.
E)flashes of light emitted from a phosphor affected by radiation.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
51
The half-life of the radioisotope 158Eu is 0.77 h.How much time is required for a 160.0-g sample of 158Eu to decay to 1.59 g?
A)6.0 h
B)4.0 h
C)3.0 h
D)2.2 h
E)5.1 h
A)6.0 h
B)4.0 h
C)3.0 h
D)2.2 h
E)5.1 h

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following types of radioactive decay has the greatest relative biological effectiveness (RBE)?
A)alpha radiation
B)electromagnetic radiation
C)beta radiation
D)gamma radiation
E)neutron radiation
A)alpha radiation
B)electromagnetic radiation
C)beta radiation
D)gamma radiation
E)neutron radiation

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
53
As a radioactive isotope decays,its rate constant
A)remains the same.
B)decreases.
C)doubles.
D)halves.
E)increases.
A)remains the same.
B)decreases.
C)doubles.
D)halves.
E)increases.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following statements is true?
A)As a radioactive isotope decays,its half-life decreases over time and its rate of disintegration remains the same.
B)As a radioactive isotope decays,its half-life remains the same and its rate of disintegration remains the same.
C)As a radioactive isotope decays,its half-life remains the same and its rate of disintegration increases over time.
D)As a radioactive isotope decays,its half-life remains the same and its rate of disintegration decreases over time.
E)As a radioactive isotope decays,its half-life decreases over time and its rate of disintegration decreases over time.
A)As a radioactive isotope decays,its half-life decreases over time and its rate of disintegration remains the same.
B)As a radioactive isotope decays,its half-life remains the same and its rate of disintegration remains the same.
C)As a radioactive isotope decays,its half-life remains the same and its rate of disintegration increases over time.
D)As a radioactive isotope decays,its half-life remains the same and its rate of disintegration decreases over time.
E)As a radioactive isotope decays,its half-life decreases over time and its rate of disintegration decreases over time.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
55
A sample of a radioactive isotope is found to have lost 19.8% of its original activity after 8.59 days.What is the decay constant of this isotope?
A)0.026 d−1
B)0.188 d−1
C)1.89 d−1
D)0.021 d−1
E)0.51 d−1
A)0.026 d−1
B)0.188 d−1
C)1.89 d−1
D)0.021 d−1
E)0.51 d−1

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
56
Consider a certain type of nucleus that has a rate constant of 2.43 × 10-2 min-1.Calculate the time required for the sample to decay to one-fourth of its initial value.
A)28.5 min
B)35.6 min
C)0.0486 min
D)57.0 min
E)2.43 min
A)28.5 min
B)35.6 min
C)0.0486 min
D)57.0 min
E)2.43 min

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
57
The rate constant for the decay of a radioactive isootope is 4.226 × 10-3 / day.What is the half-life of of this isotope?
A)328.0 days
B)410.0 days
C)82.00 days
D)164.0 days
E)none of these
A)328.0 days
B)410.0 days
C)82.00 days
D)164.0 days
E)none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
58
If a sample of the radioactive nuclide 139Cs has an activity of 0.0739 Ci,what is the instantaneous rate of decomposition of 139Cs in terms of grams per second? The mass of 139Cs is 138.9134 amu.(1 Ci = 3.700 × 1010 disintegrations/s,1 amu = 1.66054 × 10-24 g)
A)7.39×10-2 g/s
B)1.70×10-23 g/s
C)8.53×10-12 g/s
D)4.61×10-14 g/s
E)6.31×10-13 g/s
A)7.39×10-2 g/s
B)1.70×10-23 g/s
C)8.53×10-12 g/s
D)4.61×10-14 g/s
E)6.31×10-13 g/s

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
59
Iodine-131 decays by beta emission with a half-life of 8.04 days.What is the decay constant for iodine-131?
A)5.18 × 10-3/h
B)5.99 × 10-5/min
C)1.44 × 10-6/s
D)1.24× 10-1/day
E)3.35 × 10-1/h
A)5.18 × 10-3/h
B)5.99 × 10-5/min
C)1.44 × 10-6/s
D)1.24× 10-1/day
E)3.35 × 10-1/h

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following correctly represents the relationship among rads,rems,and relative biological effectiveness (RBE)?
A)rems = rads × RBE
B)rads = rems × RBE
C)rads = rems × ln(RBE)
D)rems = rads + RBE
E)none of these
A)rems = rads × RBE
B)rads = rems × RBE
C)rads = rems × ln(RBE)
D)rems = rads + RBE
E)none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
61
What is the energy change for the following nuclear bombardment reaction? (c = 3.00 × 108 m/s,1 amu = 1.66054 × 10-27 kg,1 MeV = 1.602 ×10-13 J) 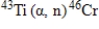 Particle
Particle
Mass (amu)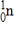
1)008665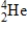
4)00260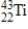
42)96852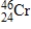
45)96837
A) 7.47×10-23MeV
B)8.76×10-22MeV
C)5.59×10-23MeV
D)0.00MeV
E)2.80×10-23MeV
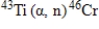 Particle
ParticleMass (amu)
1)008665
4)00260
42)96852
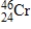
45)96837
A) 7.47×10-23MeV
B)8.76×10-22MeV
C)5.59×10-23MeV
D)0.00MeV
E)2.80×10-23MeV

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
62
A living tree contains 14C (half-life,5730 years)and has a specific activity of 750 counts per hour.A wooden artifact recovered from an archeological site gives a count of 210 counts per hour.The age of this artifact is most nearly
A)47,000 years.
B)4,500 years.
C)10,000 years.
D)22,000 years.
E)5730 years.
A)47,000 years.
B)4,500 years.
C)10,000 years.
D)22,000 years.
E)5730 years.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
63
A sample of wood from an Egyptian mummy case gives a 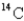 count of 8.5 cpm/gC (counts per minute per gram of carbon).How old is the wood? (The initial decay rate of
count of 8.5 cpm/gC (counts per minute per gram of carbon).How old is the wood? (The initial decay rate of 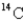
Is 15.3 cpm/g C,and its half-life is 5730 years.)
A)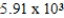 years
years
B)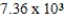 years
years
C)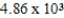 years
years
D)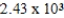 years
years
E)none of these
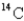 count of 8.5 cpm/gC (counts per minute per gram of carbon).How old is the wood? (The initial decay rate of
count of 8.5 cpm/gC (counts per minute per gram of carbon).How old is the wood? (The initial decay rate of 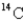
Is 15.3 cpm/g C,and its half-life is 5730 years.)
A)
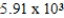 years
yearsB)
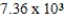 years
yearsC)
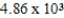 years
yearsD)
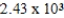 years
yearsE)none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following nuclides has the highest nuclear binding energy per nucleon?
A)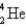
B)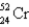
C)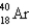
D)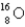
E)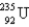
A)
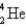
B)
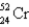
C)
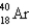
D)
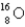
E)
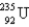

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following statements is incorrect?
A)Isotope dilution is a technique used to determine the quantity of a substance in a mixture by adding a known amount of an isotope to it.
B)Isotope dilution was used to elucidate the biological pathways of photosynthesis.
C)Isotope dilution may be used to determine the amount of vitamin B12 in food.
D)A radioactive tracer is a very small amount of a radioactive isotope added to a system used to study the chemical,physical,or biological processes of a system.
E)Neutron activation analysis is an analysis of elements in a sample based on the conversion of stable isotopes to radioactive isotopes by bombarding a sample with neutrons.
A)Isotope dilution is a technique used to determine the quantity of a substance in a mixture by adding a known amount of an isotope to it.
B)Isotope dilution was used to elucidate the biological pathways of photosynthesis.
C)Isotope dilution may be used to determine the amount of vitamin B12 in food.
D)A radioactive tracer is a very small amount of a radioactive isotope added to a system used to study the chemical,physical,or biological processes of a system.
E)Neutron activation analysis is an analysis of elements in a sample based on the conversion of stable isotopes to radioactive isotopes by bombarding a sample with neutrons.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
66
A radioactive isotope undergoes decay by emission of a positron.After 2.00 h,6.650% of the initial amount of the isotope remains undecayed.What is the half-life of this isotope?
A)45.0 min
B)30.7 min
C)90.0 min
D)15.0 min
E)60.0 min
A)45.0 min
B)30.7 min
C)90.0 min
D)15.0 min
E)60.0 min

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
67
What quantity of energy is released per gram of U-235 based on the following neutron induced fission of U-235? (c = 3.00 × 108 m/s) 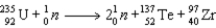 Particle
Particle
Mass (amu)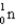
1)008665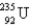
235)043922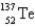
136)9253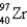
96)910950
A)7.62 × 1010 J/g U-235
B)7.62 × 1013 J/g U-235
C)1.79 × 1010 J/g U-235
D)1.79 × 1013 J/g U-235
E)3.10 × 1011 J/g U-235
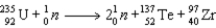 Particle
ParticleMass (amu)
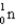
1)008665
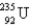
235)043922
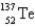
136)9253
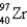
96)910950
A)7.62 × 1010 J/g U-235
B)7.62 × 1013 J/g U-235
C)1.79 × 1010 J/g U-235
D)1.79 × 1013 J/g U-235
E)3.10 × 1011 J/g U-235

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
68
A 4.50-mg sample of a newly discovered radioactive nuclide was analyzed and found to contain only 3.25 mg after a period of 25.9 h.What is the half-life of the nuclide?
A)75.0 h
B)55.2 h
C)73.5 h
D)11.4 h
E)18.9 h
A)75.0 h
B)55.2 h
C)73.5 h
D)11.4 h
E)18.9 h

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
69
How much energy is released when 2.00 metric tons of 2H2 gas undergoes nuclear fusion? (1 metric ton = 1000 kg,c = 3.00 × 108 m/s,1 amu = 1.66054 × 10-27 kg)
2H + 2H → 3He + 1n
Particle
Mass (amu)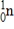
1)008665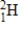
2)01400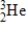
3)01603
A)6.77×10-18J
B)1.48×1017J
C)1.07×1017J
D)5.39×1010J
E)2.95×1017J
2H + 2H → 3He + 1n
Particle
Mass (amu)
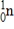
1)008665
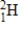
2)01400
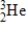
3)01603
A)6.77×10-18J
B)1.48×1017J
C)1.07×1017J
D)5.39×1010J
E)2.95×1017J

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
70
One of the first nuclear bombs based on the fission of U-235 had a maximum yield of 7.5 x1013 J.If the fission of U-235 releases 1.8 × 1013 J/mol,approximately what mass of U-235 was used in this bomb? (U-235 has a molar mass of 235.04 g/mol)
A)9.8 × 102 g U-235
B)56 g U-235
C)0.018 g U-235
D)0.0010 g U-235
E)2.3 × 102 g U-236
A)9.8 × 102 g U-235
B)56 g U-235
C)0.018 g U-235
D)0.0010 g U-235
E)2.3 × 102 g U-236

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
71
The half-life of phosphorus-33 is 25 days.How much of a 128-g sample will remain after 160 days?
A)1.5 g
B)16 g
C)1.0 g
D)8.0 g
E)4.0 g
A)1.5 g
B)16 g
C)1.0 g
D)8.0 g
E)4.0 g

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
72
The isotope 210Pb has a half-life of 22 years.What percentage of a pure 210Pb sample prepared in April 1937 remains in April 1994?
A)17%
B)26%
C)31%
D)21%
E)38%
A)17%
B)26%
C)31%
D)21%
E)38%

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
73
What assumption must be true for radiocarbon (14C)dating to be useful?
A)(14C) has the same mass as 12C.
B)A constant concentration of 14C is maintained in living plants and animals through equilibration with atmospheric levels of 14C.
C)The sample cannot have been chemically altered prior to the analysis.
D)(14C) always decays at the same rate.
E)(14C) is nonradioactive.
A)(14C) has the same mass as 12C.
B)A constant concentration of 14C is maintained in living plants and animals through equilibration with atmospheric levels of 14C.
C)The sample cannot have been chemically altered prior to the analysis.
D)(14C) always decays at the same rate.
E)(14C) is nonradioactive.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
74
Strontium-90 is produced in nuclear explosions.It can replace calcium in the bone.The half-life of 90Sr is 27.7 years.If the activity of 90Sr in the bones of an exposed person were 90 disintegrations per second,how long would it take the activity of 90Sr to decrease to 8.1 disintegrations per second?
A)96 years
B)66 years
C)49 years
D)59 years
E)75 years
A)96 years
B)66 years
C)49 years
D)59 years
E)75 years

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
75
Metastable isotopes,such as Technetium-99m and those produced from neutron activation analysis decay by what process?
A)gamma emission
B)alpha emission
C)beta emission
D)positron emission
E)all of the above
A)gamma emission
B)alpha emission
C)beta emission
D)positron emission
E)all of the above

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
76
Iodine-131,which is used to treat thyroid cancer,has a half-life of 8.04 days.How much time is required for 68% of the isotope to decay?
A)13 days
B)7 days
C)43 days
D)53 days
E)4 days
A)13 days
B)7 days
C)43 days
D)53 days
E)4 days

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
77
Relative to the sum of the masses of its constituent nucleons (neutrons + protons),the mass of a nucleus is
A)always greater.
B)sometimes the same and sometimes smaller.
C)always smaller.
D)always the same.
E)sometimes greater and sometimes smaller.
A)always greater.
B)sometimes the same and sometimes smaller.
C)always smaller.
D)always the same.
E)sometimes greater and sometimes smaller.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
78
If a tree dies and the trunk remains undisturbed for 14560 years,what percentage of original 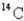 is still present? (half-life of
is still present? (half-life of 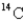
= 5730 years)
A)34.0%
B)25.0%
C)82.0%
D)17.0%
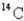 is still present? (half-life of
is still present? (half-life of 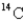
= 5730 years)
A)34.0%
B)25.0%
C)82.0%
D)17.0%

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following radioactive isotopes is not commonly used in medical applications?
A)technetium-99m
B)iodine-131
C)strontium-90
D)cobalt-60
E)thallium-201
A)technetium-99m
B)iodine-131
C)strontium-90
D)cobalt-60
E)thallium-201

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
80
The half-life of neptunium-231 is 50.0 min.How many minutes will it take for 5.0 g of this isotope to decay to 0.16 g?
A)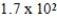 × 102 min
× 102 min
B)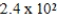 × 102 min
× 102 min
C)1.50 × 102 min
D)4.00 × 102 min
E)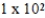 × 102 min
× 102 min
A)
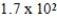 × 102 min
× 102 minB)
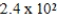 × 102 min
× 102 minC)1.50 × 102 min
D)4.00 × 102 min
E)
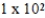 × 102 min
× 102 min
Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck


