Exam 20: Nuclear Chemistry
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
Which of the following is the most probable mode of radioactive decay for the radioactive nuclide 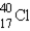 ?
?
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
A
In the radioactive decay series of plutonium-244 to form lead-208,how many alpha particles and how many beta particles are emitted per plutonium atom?
Free
(Multiple Choice)
4.7/5  (34)
(34)
Correct Answer:
C
When 25Na undergoes beta emission,what is the product nuclide?
Free
(Multiple Choice)
4.7/5  (30)
(30)
Correct Answer:
E
Which of the following nuclides has the highest nuclear binding energy per nucleon?
(Multiple Choice)
4.9/5  (39)
(39)
A nucleus located to the left of the band of stability is expected to undergo what type of nuclear decay?
(Multiple Choice)
5.0/5  (41)
(41)
A radioactive isotope decays by α emission followed by two β emissions.What is the change in the mass number and atomic number of the original isotope?
(Multiple Choice)
4.7/5  (22)
(22)
The half-life of the radioactive nuclide 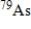 is 9.0 min.What is the activity of a 4.7-µg sample of
is 9.0 min.What is the activity of a 4.7-µg sample of 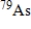 ? The molar mass of
? The molar mass of 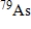 Is 78.921 g/mol.(1 Ci = 3.700 × 1010 disintegrations/s)
Is 78.921 g/mol.(1 Ci = 3.700 × 1010 disintegrations/s)
(Multiple Choice)
4.8/5  (29)
(29)
Which of the following nuclides will produce 243Am upon undergoing alpha decay?
(Multiple Choice)
4.8/5  (41)
(41)
When 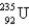 absorbs a neutron,fission occurs.One possible fission pathway is as follows:
absorbs a neutron,fission occurs.One possible fission pathway is as follows: 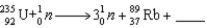 What is the missing isotope?
What is the missing isotope?
(Multiple Choice)
4.8/5  (28)
(28)
Use the following table to assist in answering the question below.
Nuclide
Half-Life
Uranium-238
4)51 × 109 years
Uranium-234
2)48 × 105 years
Thorium-230
8)0 × 104 years
Radium-226
1)62 × 103 years
Lead-210
20)4 years
The rate constant for the decay of unstable nuclide X by alpha-particle emission is 1.17 × 10-6 / day.What is the identity of X?
(Multiple Choice)
4.8/5  (36)
(36)
A living tree contains 14C (half-life,5730 years)and has a specific activity of 750 counts per hour.A wooden artifact recovered from an archeological site gives a count of 210 counts per hour.The age of this artifact is most nearly
(Multiple Choice)
4.8/5  (39)
(39)
If a tree dies and the trunk remains undisturbed for 14560 years,what percentage of original 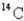 is still present? (half-life of
is still present? (half-life of 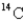 = 5730 years)
= 5730 years)
(Multiple Choice)
4.9/5  (40)
(40)
How much energy is released when 2.00 metric tons of 2H2 gas undergoes nuclear fusion? (1 metric ton = 1000 kg,c = 3.00 × 108 m/s,1 amu = 1.66054 × 10-27 kg)
2H + 2H → 3He + 1n
Particle
Mass (amu) 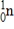
1)008665
1)008665 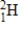
2)01400
2)01400 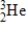
3)01603
3)01603
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following corresponds to the most rapid nuclear decay?
(Multiple Choice)
4.8/5  (31)
(31)
The commonly used radioactive isotopes radium-226,radon-222,and cobalt-60 are used in _____.
(Multiple Choice)
4.8/5  (31)
(31)
If 1 mol of oxygen-16 were formed from protons and neutrons,0.1366 g of mass would be lost.What can best account for this loss?
(Multiple Choice)
4.8/5  (33)
(33)
The half-life of phosphorus-33 is 25 days.How much of a 128-g sample will remain after 160 days?
(Multiple Choice)
4.8/5  (39)
(39)
When the radioactive nuclide 198Po undergoes alpha emission,what is the product nuclide?
(Multiple Choice)
4.9/5  (28)
(28)
Showing 1 - 20 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)