Deck 30: Nuclear Energy and Elementary Particles: Part A
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/37
Play
Full screen (f)
Deck 30: Nuclear Energy and Elementary Particles: Part A
1
1010 Bq = ______ Ci?
A) 2.7 × 10-11
B) 3.7
C) 0.27
D) 3.7 × 1010
A) 2.7 × 10-11
B) 3.7
C) 0.27
D) 3.7 × 1010
0.27
2
A pure sample of 226Ra contains 3.0 × 1014 atoms of the isotope.If the half-life of 226Ra = 1.6 × 103 years,what is the activity of this sample?
A) 13 × 1010 decays/yr
B) 9.4 × 1010 decays/yr
C) 6.7 × 109 decays/yr
D) 8.7 × 1010decays/yr
A) 13 × 1010 decays/yr
B) 9.4 × 1010 decays/yr
C) 6.7 × 109 decays/yr
D) 8.7 × 1010decays/yr
13 × 1010 decays/yr
3
Approximately how many half-life periods must elapse if the activity of a radioactive isotope sample is to be reduced to 0.03 of the original value?
A) 5
B) 3
C) 8
D) 60
A) 5
B) 3
C) 8
D) 60
5
4
A pure sample of 226Ra contains 3.0 × 1014 atoms of the isotope.If the half-life of 226Ra = 1.6 × 103 years,what is the decay rate of this sample? (1 Ci = 3.7 × 1010 decays/s)
A) 2.7 × 10−12 Ci
B) 11 × 10−8 Ci
C) 3.4 × 10−10 Ci
D) 7.4 × 10−8 Ci
A) 2.7 × 10−12 Ci
B) 11 × 10−8 Ci
C) 3.4 × 10−10 Ci
D) 7.4 × 10−8 Ci

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
5
The half-life of radioactive Technetium-99 is 6.0 hours.Find the number of 99Tc nuclei necessary to produce a sample of activity 18 µCi.(1 Ci = 3.7 × 1010 decays/second)
A) 1.2 × 109
B) 3.4 × 1011
C) 8.0 × 108
D) 2.1 × 1010
A) 1.2 × 109
B) 3.4 × 1011
C) 8.0 × 108
D) 2.1 × 1010

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
6
Approximately how many radioactive atoms are present in a tritium sample with an activity of 0.6 × 10−6 Ci and a half-life of 12.3 years? (1 Ci = 3.7 × 1010 decays/s)
A) 4 × 1010
B) 2 × 108
C) 7 × 108
D) 1 × 1013
A) 4 × 1010
B) 2 × 108
C) 7 × 108
D) 1 × 1013

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
7
The isotope 64Zn has a nuclear radius of 4.8 × 10−15 m.Approximately what is the nuclear radius of the isotope 125Te?
A) 2.0 × 10−15 m
B) 3.6 × 10−15 m
C) 2.7 × 10−15 m
D) 6.0 × 10−15 m
A) 2.0 × 10−15 m
B) 3.6 × 10−15 m
C) 2.7 × 10−15 m
D) 6.0 × 10−15 m

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
8
The relationship 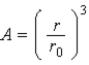
,where A is the mass number of a nucleus and r is it radius,implies which of the following about nuclei?
A) Nuclei are cubic.
B) Different nuclei have the same density.
C) Neutrons in nuclei attract each other even though they have no charge.
D) Nuclei tend to have the same ratio of protons to neutrons.
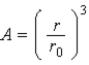
,where A is the mass number of a nucleus and r is it radius,implies which of the following about nuclei?
A) Nuclei are cubic.
B) Different nuclei have the same density.
C) Neutrons in nuclei attract each other even though they have no charge.
D) Nuclei tend to have the same ratio of protons to neutrons.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
9
Over the course of 6 hours,15% of a radioactive material decays.What is its half-life?
A) 12.8 hrs
B) 25.6 hrs
C) 4.1 hrs
D) 68.6 hrs
A) 12.8 hrs
B) 25.6 hrs
C) 4.1 hrs
D) 68.6 hrs

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
10
Neglecting recoil of the gold nucleus,how much kinetic energy must an alpha particle (charge = 2 × 1.6 × 10−19 C)have to approach to within 1.95 × 10−14 m of a gold nucleus (charge = 79 × 1.6 × 10−19 C)? (ke = 8.99 × 109 N⋅m2/C2 and 1 MeV = 1.6 × 10−13 J)
A) 14.6 MeV
B) 11.7 MeV
C) 18.2 MeV
D) 22.7 MeV
A) 14.6 MeV
B) 11.7 MeV
C) 18.2 MeV
D) 22.7 MeV

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
11
The isotope 64Zn has a nuclear radius of 4.8 ×10−15 m.Which of the following is the mass number of an isotope for which the nuclear radius is 6.8 ×10−15 m?
A) 182
B) 125
C) 145
D) 96
A) 182
B) 125
C) 145
D) 96

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
12
Tritium has a half-life of 12.3 years.What proportion of its original radioactivity will a sample have after 6 years?
A) 0.55
B) 0.60
C) 0.84
D) 0.71
A) 0.55
B) 0.60
C) 0.84
D) 0.71

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
13
If a fossil bone is found to contain 1/16th as much Carbon-14 as the bone of a living animal,what is the approximate age of the fossil? (half-life of 14C = 5730 years)
A) 7640 years
B) 22,900 years
C) 45,800 years
D) 17,200 years
A) 7640 years
B) 22,900 years
C) 45,800 years
D) 17,200 years

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
14
Tritium has a half-life of 12.3 years.How many years will elapse when the radioactivity of a tritium sample diminishes to 30% of its original value?
A) 29 years
B) 21 years
C) 86 years
D) 57 years
A) 29 years
B) 21 years
C) 86 years
D) 57 years

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
15
An ancient building was known to have been built 4000 years ago.Approximately what proportion of Carbon-14 atoms are still in the building's wooden framing compared to the number which were present at the time of its construction? (half life of 14C = 5730 years)
A) 0.696
B) 0.616
C) 0.500
D) 0.425
A) 0.696
B) 0.616
C) 0.500
D) 0.425

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
16
Sample #1 is made from an isotope with decay constant 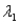
And sample #2 is made from an isotope with decay constant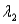
,where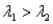
)Which of the following statements must be true?
A) The activity of sample #2 is greater than that of sample #1.
B) The activity of sample #1 is greater than that of sample #2.
C) The half-life exhibited for sample #1 is greater than that of sample #2.
D) The half-life exhibited for sample #2 is greater than that for sample #1
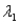
And sample #2 is made from an isotope with decay constant
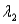
,where
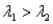
)Which of the following statements must be true?
A) The activity of sample #2 is greater than that of sample #1.
B) The activity of sample #1 is greater than that of sample #2.
C) The half-life exhibited for sample #1 is greater than that of sample #2.
D) The half-life exhibited for sample #2 is greater than that for sample #1

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
17
If there are 146 neutrons in 238U,how many neutrons are found in the nucleus of 233U?
A) 147
B) 145
C) 143
D) 141
A) 147
B) 145
C) 143
D) 141

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
18
Certain stars at the end of their lives are thought to collapse,combining their protons and electrons together to form a neutron star.Such a star could be thought of as a giant atomic nucleus.If a star of mass equal to M = 4.00 × 1030 kg collapsed into neutrons (mn = 1.67 × 10−27 kg),what would be the radius of such a star? (Hint: r = r0A1/3,where r0 = 1.20 × 10−15 m. )
A) 12.7 km
B) 25.4 km
C) 16.1 km
D) 18.5 km
A) 12.7 km
B) 25.4 km
C) 16.1 km
D) 18.5 km

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
19
Tritium is radioactive with a half-life of 12.33 years decaying into 3He with low-energy electron emission.If we have a sample of 2.35 × 1019 tritium atoms,what is its activity in decays/second? (1 year = 3.16 × 107 s)
A) 6.64 × 107/second
B) 5.35 × 109 /second
C) 3.69 × 108/second
D) 4.18 × 1010/second
A) 6.64 × 107/second
B) 5.35 × 109 /second
C) 3.69 × 108/second
D) 4.18 × 1010/second

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
20
A radioactive material initially is observed to have an activity of 1000 decays/sec.If nine hours later it is observed to have an activity of 125 decays/s,what is its half-life?
A) 1/2 hour
B) 8 hours
C) 3 hours
D) 1 hour
A) 1/2 hour
B) 8 hours
C) 3 hours
D) 1 hour

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
21
The half-life of 19N is 0.42 s.What is the decay constant for this isotope?
A) 1.7 × 10-11 Ci
B) 1.7 s-1
C) 1.1 s-1
D) The decay constant is not defined for a half-life of less than one second.
A) 1.7 × 10-11 Ci
B) 1.7 s-1
C) 1.1 s-1
D) The decay constant is not defined for a half-life of less than one second.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
22
Tritium (3H)has a half-life of 12.3 years and releases 0.0186 MeV energy per decay.What is the rate at which energy is released for a 3.0-gram sample of tritium? (NA = 6.02 × 1023 mol-1,1 year = 3.16 × 107 s,1 MeV = 1.6 × 10-13 J,and mt = 3.01605 u)
A) 3.2 W
B) 1.1 W
C) 9.6 W
D) 0.33 W
A) 3.2 W
B) 1.1 W
C) 9.6 W
D) 0.33 W

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
23
Tritium (3H)has a half-life of 12.3 years and releases 0.0186 MeV energy per decay.What is the activity of a 9.0-gram sample of tritium? (NA = 6.02 × 1023 mol-1,1 year = 3.16 × 107 s,1 MeV = 1.6 × 10-13 J,and mt = 3.01605 u)
A) 1.3 × 1016 Bq
B) 1.1 × 1015 Bq
C) 3.6 × 1014 Bq
D) 3.2 × 1015 Bq
A) 1.3 × 1016 Bq
B) 1.1 × 1015 Bq
C) 3.6 × 1014 Bq
D) 3.2 × 1015 Bq

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
24
What particle is emitted when 236Pa decays to 236U? (The atomic numbers of Pa and U are,respectively,91 and 92. )
A) beta (positron)
B) alpha
C) gamma quantum
D) beta (electron)
A) beta (positron)
B) alpha
C) gamma quantum
D) beta (electron)

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
25
About what fraction of the initial activity of a radioactive source remains after 10 half-lives?
A) 1.0 × 10-10
B) 9.8 × 10-4
C) 5.0 × 10-2
D) 3.9 × 10-3
A) 1.0 × 10-10
B) 9.8 × 10-4
C) 5.0 × 10-2
D) 3.9 × 10-3

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
26
A deuteron is captured by an oxygen-16 atom which in turn emits a proton.What is the element and mass number of the product isotope?
A) oxygen-15
B) fluorine-15
C) nitrogen-15
D) oxygen-17
A) oxygen-15
B) fluorine-15
C) nitrogen-15
D) oxygen-17

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
27
The energy released from a nuclear reaction is equal to:
A) energy associated with momentum conservation.
B) the exothermic endothermy.
C) energy associated with the change in mass.
D) the total charge involved.
A) energy associated with momentum conservation.
B) the exothermic endothermy.
C) energy associated with the change in mass.
D) the total charge involved.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
28
What energy must be added or given off in a reaction where a helium atom is separated into two hydrogen atoms and two neutrons? (Atomic masses for each: hydrogen,1.007825 u;neutron,1.008665 u;helium,4.002602 u;also,1 u = 931.5 MeV/c2. )
A) 28.3 MeV given off
B) 20.7 MeV added
C) 20.7 MeV given off
D) 28.3 MeV added
A) 28.3 MeV given off
B) 20.7 MeV added
C) 20.7 MeV given off
D) 28.3 MeV added

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
29
What is the energy released for the reaction where the products are 0.0051 u less than the reactants? (1 u = 931.5 MeV/c2)
A) 7.6 MeV
B) 5.2 MeV
C) 4.8 MeV
D) 8.5 MeV
A) 7.6 MeV
B) 5.2 MeV
C) 4.8 MeV
D) 8.5 MeV

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
30
Calculate the binding energy per nucleon of the 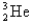 Nucleus,given that the mass of this nucleus is 3.016029 u.(mp = 1.007276 u,mn = 1.008665,and 1 u = 931.5 MeV/c2)
Nucleus,given that the mass of this nucleus is 3.016029 u.(mp = 1.007276 u,mn = 1.008665,and 1 u = 931.5 MeV/c2)
A) 2.66 MeV/nucleon
B) 2.86 MeV/nucleon
C) 2.45 MeV/nucleon
D) 2.23 MeV/nucleon
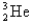 Nucleus,given that the mass of this nucleus is 3.016029 u.(mp = 1.007276 u,mn = 1.008665,and 1 u = 931.5 MeV/c2)
Nucleus,given that the mass of this nucleus is 3.016029 u.(mp = 1.007276 u,mn = 1.008665,and 1 u = 931.5 MeV/c2)
A) 2.66 MeV/nucleon
B) 2.86 MeV/nucleon
C) 2.45 MeV/nucleon
D) 2.23 MeV/nucleon

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
31
What particle is emitted when 20Na decays to 20Ne? (The atomic numbers of Na and Ne are,respectively,11 and 10. )
A) beta (positron)
B) gamma quantum
C) alpha
D) beta (electron)
A) beta (positron)
B) gamma quantum
C) alpha
D) beta (electron)

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
32
What is the energy released (or gained signified by the minus sign)value of the reaction 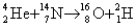
? The mass of the alpha particle is 4.002602 u,of the nitrogen is 14.003074 u,of the oxygen is 15.994915 u,and of the hydrogen is 2.014102 u.(1 u = 931.5 MeV/c2)
A) -3.11 MeV
B) -3.34 MeV
C) 3.11 MeV
D) 3.34 MeV
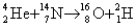
? The mass of the alpha particle is 4.002602 u,of the nitrogen is 14.003074 u,of the oxygen is 15.994915 u,and of the hydrogen is 2.014102 u.(1 u = 931.5 MeV/c2)
A) -3.11 MeV
B) -3.34 MeV
C) 3.11 MeV
D) 3.34 MeV

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
33
In the reaction 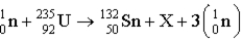
,what are the mass number and the atomic number of the product designated by X?
A) 101,42
B) 102,44
C) 102,42
D) not given
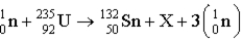
,what are the mass number and the atomic number of the product designated by X?
A) 101,42
B) 102,44
C) 102,42
D) not given

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
34
The isotope 238U,which starts one of the natural radioactive series,decays first by alpha decay followed by two negative beta decays.At this point,what is the resulting isotope?
A) 234Th (Z = 90 for Th. )
B) 236U
C) 238U
D) Some other uranium isotope not given above.
A) 234Th (Z = 90 for Th. )
B) 236U
C) 238U
D) Some other uranium isotope not given above.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
35
One of the nuclear reactions that occurs is 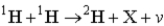 )What does the X represent in this equation?
)What does the X represent in this equation?
A)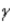
B)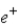
C)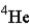
D)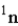
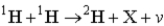 )What does the X represent in this equation?
)What does the X represent in this equation?A)
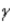
B)
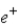
C)
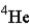
D)
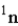

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
36
An alpha particle (mass = 6.68 × 10−27 kg)is emitted from a radioactive nucleus with an energy of 6.22 MeV.How fast is the alpha particle moving in m/s? (1 MeV = 1.6 × 10−13 J)
A) 3.70 × 106 m/s
B) 1.73 × 107 m/s
C) 1.55 × 107 m/s
D) 1.85 × 106 m/s
A) 3.70 × 106 m/s
B) 1.73 × 107 m/s
C) 1.55 × 107 m/s
D) 1.85 × 106 m/s

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following methods of decay does not result in an element different from that of the original parent nucleus?
A) alpha followed by negative beta followed by another negative beta
B) alpha followed by beta
C) alpha followed by gamma followed by another gamma
D) alpha followed by positive beta followed by another positive beta
A) alpha followed by negative beta followed by another negative beta
B) alpha followed by beta
C) alpha followed by gamma followed by another gamma
D) alpha followed by positive beta followed by another positive beta

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck


