Exam 30: Nuclear Energy and Elementary Particles: Part A
Exam 1: Introduction60 Questions
Exam 1: Introduction: Part A47 Questions
Exam 2: Motion in One Dimension64 Questions
Exam 2: Motion in One Dimension: Part A42 Questions
Exam 3: Vectors and Two-Dimensional Motion74 Questions
Exam 3: Vectors and Two-Dimensional Motion: Part A64 Questions
Exam 4: The Laws of Motion93 Questions
Exam 4: The Laws of Motion: Part A69 Questions
Exam 5: Energy84 Questions
Exam 5: Energy: Part A32 Questions
Exam 6: Momentum and Collisions83 Questions
Exam 6: Momentum and Collisions: Part A61 Questions
Exam 7: Rotational Motion and the Law of Gravity84 Questions
Exam 7: Rotational Motion and the Law of Gravity: Part A48 Questions
Exam 8: Rotational Equilibrium and Rotational Dynamics60 Questions
Exam 8: Rotational Equilibrium and Rotational Dynamics: Part A61 Questions
Exam 9: Solids and Fluids78 Questions
Exam 9: Solids and Fluids: Part A46 Questions
Exam 10: Thermal Physics82 Questions
Exam 10: Thermal Physics: Part A56 Questions
Exam 11: Energy in Thermal Processes Heat and Internal Energy82 Questions
Exam 11: Energy in Thermal Processes Heat and Internal Energy: Part A54 Questions
Exam 12: The Laws of Thermodynamics Work in Thermodynamic Processes70 Questions
Exam 12: The Laws of Thermodynamics Work in Thermodynamic Processes: Part A40 Questions
Exam 13: Vibrations and Waves83 Questions
Exam 13: Vibrations and Waves: Part A48 Questions
Exam 14: Sound81 Questions
Exam 14: Sound: Part A67 Questions
Exam 15: Electric Forces and Electric Fields81 Questions
Exam 15: Electric Forces and Electric Fields: Part A42 Questions
Exam 16: Electrical Energy and Capacitance81 Questions
Exam 16: Electrical Energy and Capacitance: Part A33 Questions
Exam 17: Current and Resistance Electric Current83 Questions
Exam 17: Current and Resistance Electric Current: Part A37 Questions
Exam 18: Direct-Current Circuits Sources of EMF77 Questions
Exam 18: Direct-Current Circuits Sources of EMF: Part A54 Questions
Exam 19: Magnetism Magnets82 Questions
Exam 19: Magnetism Magnets: Part A67 Questions
Exam 20: Induced Voltages and Inductance83 Questions
Exam 20: Induced Voltages and Inductance: Part A46 Questions
Exam 21: Alternating-Current Circuits and Electromagnetic Waves98 Questions
Exam 21: Alternating-Current Circuits and Electromagnetic Waves: Part A32 Questions
Exam 22: Reflection and Refraction of Light81 Questions
Exam 22: Reflection and Refraction of Light: Part A49 Questions
Exam 23: Mirrors and Lenses82 Questions
Exam 23: Mirrors and Lenses: Part A31 Questions
Exam 24: Wave Optics88 Questions
Exam 24: Wave Optics: Part A71 Questions
Exam 25: Optical Instruments79 Questions
Exam 25: Optical Instruments: Part A59 Questions
Exam 26: Relativity62 Questions
Exam 26: Relativity: Part A29 Questions
Exam 27: Quantum Physics Blackbody Radiation and Plancks Hypothesis79 Questions
Exam 27: Quantum Physics Blackbody Radiation and Plancks Hypothesis: Part A52 Questions
Exam 28: Atomic Physics71 Questions
Exam 28: Atomic Physics: Part A38 Questions
Exam 29: Nuclear Physics75 Questions
Exam 29: Nuclear Physics: Part A43 Questions
Exam 30: Nuclear Energy and Elementary Particles88 Questions
Exam 30: Nuclear Energy and Elementary Particles: Part A37 Questions
Exam 31: Particle Collisions, Mediating Photons, and Quark Structures: Exploring the Fundamentals of Physics16 Questions
Select questions type
A radioactive material initially is observed to have an activity of 1000 decays/sec.If nine hours later it is observed to have an activity of 125 decays/s,what is its half-life?
Free
(Multiple Choice)
4.7/5  (33)
(33)
Correct Answer:
C
What is the energy released for the reaction where the products are 0.0051 u less than the reactants? (1 u = 931.5 MeV/c2)
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
C
Calculate the binding energy per nucleon of the 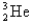 Nucleus,given that the mass of this nucleus is 3.016029 u.(mp = 1.007276 u,mn = 1.008665,and 1 u = 931.5 MeV/c2)
Nucleus,given that the mass of this nucleus is 3.016029 u.(mp = 1.007276 u,mn = 1.008665,and 1 u = 931.5 MeV/c2)
Free
(Multiple Choice)
4.7/5  (25)
(25)
Correct Answer:
D
What energy must be added or given off in a reaction where a helium atom is separated into two hydrogen atoms and two neutrons? (Atomic masses for each: hydrogen,1.007825 u;neutron,1.008665 u;helium,4.002602 u;also,1 u = 931.5 MeV/c2. )
(Multiple Choice)
4.8/5  (42)
(42)
The isotope 64Zn has a nuclear radius of 4.8 × 10−15 m.Approximately what is the nuclear radius of the isotope 125Te?
(Multiple Choice)
4.8/5  (40)
(40)
Approximately how many radioactive atoms are present in a tritium sample with an activity of 0.6 × 10−6 Ci and a half-life of 12.3 years? (1 Ci = 3.7 × 1010 decays/s)
(Multiple Choice)
4.8/5  (36)
(36)
An alpha particle (mass = 6.68 × 10−27 kg)is emitted from a radioactive nucleus with an energy of 6.22 MeV.How fast is the alpha particle moving in m/s? (1 MeV = 1.6 × 10−13 J)
(Multiple Choice)
4.9/5  (38)
(38)
Tritium (3H)has a half-life of 12.3 years and releases 0.0186 MeV energy per decay.What is the rate at which energy is released for a 3.0-gram sample of tritium? (NA = 6.02 × 1023 mol-1,1 year = 3.16 × 107 s,1 MeV = 1.6 × 10-13 J,and mt = 3.01605 u)
(Multiple Choice)
4.7/5  (38)
(38)
What is the energy released (or gained signified by the minus sign)value of the reaction 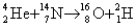 ? The mass of the alpha particle is 4.002602 u,of the nitrogen is 14.003074 u,of the oxygen is 15.994915 u,and of the hydrogen is 2.014102 u.(1 u = 931.5 MeV/c2)
? The mass of the alpha particle is 4.002602 u,of the nitrogen is 14.003074 u,of the oxygen is 15.994915 u,and of the hydrogen is 2.014102 u.(1 u = 931.5 MeV/c2)
(Multiple Choice)
4.9/5  (34)
(34)
The relationship 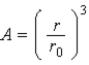 ,where A is the mass number of a nucleus and r is it radius,implies which of the following about nuclei?
,where A is the mass number of a nucleus and r is it radius,implies which of the following about nuclei?
(Multiple Choice)
5.0/5  (33)
(33)
If there are 146 neutrons in 238U,how many neutrons are found in the nucleus of 233U?
(Multiple Choice)
4.8/5  (34)
(34)
The isotope 238U,which starts one of the natural radioactive series,decays first by alpha decay followed by two negative beta decays.At this point,what is the resulting isotope?
(Multiple Choice)
4.9/5  (30)
(30)
Tritium is radioactive with a half-life of 12.33 years decaying into 3He with low-energy electron emission.If we have a sample of 2.35 × 1019 tritium atoms,what is its activity in decays/second? (1 year = 3.16 × 107 s)
(Multiple Choice)
4.9/5  (38)
(38)
The half-life of 19N is 0.42 s.What is the decay constant for this isotope?
(Multiple Choice)
4.8/5  (26)
(26)
Which of the following methods of decay does not result in an element different from that of the original parent nucleus?
(Multiple Choice)
4.7/5  (40)
(40)
In the reaction 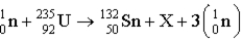 ,what are the mass number and the atomic number of the product designated by X?
,what are the mass number and the atomic number of the product designated by X?
(Multiple Choice)
4.9/5  (37)
(37)
A deuteron is captured by an oxygen-16 atom which in turn emits a proton.What is the element and mass number of the product isotope?
(Multiple Choice)
4.9/5  (33)
(33)
Approximately how many half-life periods must elapse if the activity of a radioactive isotope sample is to be reduced to 0.03 of the original value?
(Multiple Choice)
4.9/5  (41)
(41)
Certain stars at the end of their lives are thought to collapse,combining their protons and electrons together to form a neutron star.Such a star could be thought of as a giant atomic nucleus.If a star of mass equal to M = 4.00 × 1030 kg collapsed into neutrons (mn = 1.67 × 10−27 kg),what would be the radius of such a star? (Hint: r = r0A1/3,where r0 = 1.20 × 10−15 m. )
(Multiple Choice)
4.9/5  (29)
(29)
Showing 1 - 20 of 37
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)