Deck 12: The Laws of Thermodynamics Work in Thermodynamic Processes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/70
Play
Full screen (f)
Deck 12: The Laws of Thermodynamics Work in Thermodynamic Processes
1
A 2-mol ideal gas system undergoes an adiabatic process where it expands and does 20 J of work on its environment.How much heat is received by the system?
A) −20 J
B) zero
C) +10 J
D) +20 J
E) −10 J
A) −20 J
B) zero
C) +10 J
D) +20 J
E) −10 J
zero
2
In an isothermal process for an ideal gas system (where the internal energy doesn't change),which of the following choices best corresponds to the value of the work done on the system?
A) its heat intake
B) twice its heat intake
C) the negative of its heat intake
D) twice the negative of its heat intake
A) its heat intake
B) twice its heat intake
C) the negative of its heat intake
D) twice the negative of its heat intake
the negative of its heat intake
3
A 2-mol ideal gas system undergoes an adiabatic process where it expands and does 16 J of work on its environment.What is its change in internal energy?
A) −16 J
B) −8 J
C) zero
D) +16 J
E) +8 J
A) −16 J
B) −8 J
C) zero
D) +16 J
E) +8 J
−16 J
4
A system is acted on by its surroundings in such a way that it receives 70 J of heat while simultaneously doing 40 J of work.What is its net change in internal energy?
A) 110 J
B) 30 J
C) zero
D) −30 J
E) −110 J
A) 110 J
B) 30 J
C) zero
D) −30 J
E) −110 J

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
5
A closed 2.0-L container holds 3.0 mol of an ideal gas.If 180 J of heat is added,what is the change in internal energy of the system?
A) zero
B) 130 J
C) 155 J
D) 180 J
E) 230 J
A) zero
B) 130 J
C) 155 J
D) 180 J
E) 230 J

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
6
Area on a P-V diagram has units associated with:
A) energy.
B) momentum.
C) temperature.
D) change in temperature.
A) energy.
B) momentum.
C) temperature.
D) change in temperature.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
7
In an isobaric process 5 × 104 J of work is done on a quantity of gas while its volume changes from 2.8 m3 to 1.8 m3.What is the pressure during this process?
A) 4.4 × 104 Pa
B) 3.8 × 104 Pa
C) 5.0 × 104 Pa
D) 5.6 × 104 Pa
E) 6.2 × 104 Pa
A) 4.4 × 104 Pa
B) 3.8 × 104 Pa
C) 5.0 × 104 Pa
D) 5.6 × 104 Pa
E) 6.2 × 104 Pa

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
8
The adiabatic index of a gas is given by which of the following?
A) CP /CV
B) CV /CP
C) CP − CV
D) CP + CV
A) CP /CV
B) CV /CP
C) CP − CV
D) CP + CV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
9
In the first law of thermodynamics, 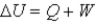
,W is positive when
A) the work is being done on the environment by the system.
B) the work is being done on the system by the environment.
C) the work is being done on the environment by the system,and the temperature of the system goes up.
D) the work is being done on the system by the environment,and the temperature of the system goes up.
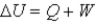
,W is positive when
A) the work is being done on the environment by the system.
B) the work is being done on the system by the environment.
C) the work is being done on the environment by the system,and the temperature of the system goes up.
D) the work is being done on the system by the environment,and the temperature of the system goes up.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
10
A 5-mol ideal gas system undergoes an adiabatic free expansion (a rapid expansion into a vacuum),going from an initial volume of 30 L to a final volume of 50 L.How much work is done on the system during this adiabatic free expansion?
A) −150 J
B) −30 J
C) zero
D) +150 J
E) +30 J
A) −150 J
B) −30 J
C) zero
D) +150 J
E) +30 J

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
11
According to the first law of thermodynamics,the sum of the heat gained by a system and the work done on that same system is equivalent to which of the following?
A) entropy change
B) internal energy change
C) temperature change
D) specific heat
A) entropy change
B) internal energy change
C) temperature change
D) specific heat

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
12
The volume of an ideal gas changes from 0.3 to 0.52 m3 although its pressure remains constant at 58,000 Pa.What work is done on the system by its environment?
A) -12,760 J
B) 12,760 J
C) -17,400 J
D) 17,400 J
E) -30,160 J
A) -12,760 J
B) 12,760 J
C) -17,400 J
D) 17,400 J
E) -30,160 J

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
13
If an ideal gas does positive work on its surroundings,we may assume,with regard to the gas:
A) temperature increases.
B) volume increases.
C) pressure increases.
D) internal energy decreases.
A) temperature increases.
B) volume increases.
C) pressure increases.
D) internal energy decreases.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following increases the internal energy of a solid metal rod?
A) raising it to a greater height
B) throwing it through the air
C) having the rod conduct heat
D) having the rod absorb heat
A) raising it to a greater height
B) throwing it through the air
C) having the rod conduct heat
D) having the rod absorb heat

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
15
An adiabatic expansion refers to the fact that:
A) no heat is transferred between a system and its surroundings.
B) the pressure remains constant.
C) the temperature remains constant.
D) the volume remains constant.
A) no heat is transferred between a system and its surroundings.
B) the pressure remains constant.
C) the temperature remains constant.
D) the volume remains constant.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
16
A quantity of monatomic ideal gas expands adiabatically from a volume of 2.4 liters to 6.7 liters.If the initial pressure is P0,what is the final pressure?
A) 2.79 P0
B) 5.53 P0
C) 5.58 P0
D) 0.18 P0
E) 0.36 P0
A) 2.79 P0
B) 5.53 P0
C) 5.58 P0
D) 0.18 P0
E) 0.36 P0

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
17
In an isovolumetric process by an ideal gas,the system's heat gain is equivalent to a change in:
A) temperature.
B) volume.
C) pressure.
D) internal energy.
A) temperature.
B) volume.
C) pressure.
D) internal energy.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
18
On a P-V diagram,an ____ process is represented by a horizontal line.
A) isobaric
B) isothermal
C) isovolumetric
D) adiabatic
A) isobaric
B) isothermal
C) isovolumetric
D) adiabatic

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
19
During an isobaric process which one of the following does not change?
A) volume
B) temperature
C) internal energy
D) pressure
A) volume
B) temperature
C) internal energy
D) pressure

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
20
A 2.0-mol ideal gas system is maintained at a constant volume of 4.0 L.If 100 J of heat is added,what is the work done on the system?
A) zero
B) 5 J
C) −3.3 J
D) 20 J
E) 3.3 J
A) zero
B) 5 J
C) −3.3 J
D) 20 J
E) 3.3 J

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
21
System 1 is 3.6 moles of a monatomic ideal gas held at a constant volume as it undergoes a temperature increase.System 2 is 3.6 moles of a diatomic ideal gas held at constant volume as it also undergoes a temperature increase.The thermal energy absorbed by System 1 is 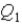
,and the thermal energy absorbed by System 2 is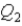
As both systems undergo a temperature increase of 74.6 K.What is the ratio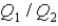
?
A) 1.00
B) 0.60
C) 1.67
D) The volumes of the systems need to be known before the ratio can be found.
E) 2.50
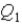
,and the thermal energy absorbed by System 2 is
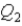
As both systems undergo a temperature increase of 74.6 K.What is the ratio
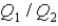
?
A) 1.00
B) 0.60
C) 1.67
D) The volumes of the systems need to be known before the ratio can be found.
E) 2.50

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
22
A heat engine operating between a pair of hot and cold reservoirs with respective temperatures of 500 K and 300 K will have what maximum efficiency?
A) 40%
B) 60%
C) 30%
D) 35%
E) 16%
A) 40%
B) 60%
C) 30%
D) 35%
E) 16%

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
23
According to the second law of thermodynamics,which of the following applies to the heat received from a high temperature reservoir by a heat engine operating in a complete cycle?
A) must be completely converted to work
B) equals the entropy increase
C) converted completely into internal energy
D) cannot be completely converted to work
A) must be completely converted to work
B) equals the entropy increase
C) converted completely into internal energy
D) cannot be completely converted to work

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
24
An electrical power plant manages to send 87% of the heat produced in the burning of fossil fuel into the water-to-steam conversion.Of the heat carried by the steam,40% is converted to the mechanical energy of the spinning turbine.Which of the following choices best describes the overall efficiency of the heat-to-work conversion in the plant (as a percentage)?
A) greater than 87%
B) 64%
C) less than 40%
D) 40%
E) 87%
A) greater than 87%
B) 64%
C) less than 40%
D) 40%
E) 87%

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
25
In which system is heat usually transferred from the cooler part to the warmer part?
A) a stove as it heats up water
B) a refrigerator that is running
C) an electric fan that is running
D) none of the above,because it is impossible to transfer heat in this manner
A) a stove as it heats up water
B) a refrigerator that is running
C) an electric fan that is running
D) none of the above,because it is impossible to transfer heat in this manner

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
26
When gasoline is burned,it gives off 47,000 J/g of heat energy.If an automobile uses 12.7 kg of gasoline per hour with an efficiency of 24%,what is the average horsepower output of the engine? (1 hp = 746 W)
A) 53 hp
B) 126 hp
C) 42 hp
D) 32 hp
E) 63 hp
A) 53 hp
B) 126 hp
C) 42 hp
D) 32 hp
E) 63 hp

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
27
System 1 is 3.1 moles of a monatomic ideal gas held at a constant volume of 2.51 L as it undergoes a temperature increase.System 2 is 3.1 moles of a diatomic ideal gas held at a constant volume of 2.51 L as it undergoes a temperature increase equal to the temperature increase of System 1.The pressure increase for system 1 is 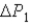
And for System 2 is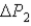
During the temperature increases.What is the ratio of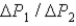
?
A) 1.00
B) 0.60
C) 1.67
D) 2.50
E) 4.50
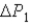
And for System 2 is
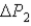
During the temperature increases.What is the ratio of
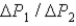
?
A) 1.00
B) 0.60
C) 1.67
D) 2.50
E) 4.50

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
28
An electrical generating plant operates at a boiler temperature of 224°C and exhausts the unused heat into a nearby river at 21°C.What is the maximum theoretical efficiency of the plant? (0°C = 273 K)
A) 17%
B) 7%
C) 64%
D) 41%
E) 74%
A) 17%
B) 7%
C) 64%
D) 41%
E) 74%

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
29
A heat engine exhausts 3,200 J of heat while performing 1,500 J of useful work.What is the efficiency of the engine?
A) 68%
B) 32%
C) 16%
D) 50%
E) 84%
A) 68%
B) 32%
C) 16%
D) 50%
E) 84%

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
30
A turbine takes in 1,200-K steam and exhausts the steam at a temperature of 300 K.What is the maximum theoretical efficiency of this system?
A) 56%
B) 42%
C) 75%
D) 32%
E) 24%
A) 56%
B) 42%
C) 75%
D) 32%
E) 24%

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
31
A heat engine receives 7,100 J of heat from its combustion process and loses 3,100 J through the exhaust and friction.What is its efficiency?
A) 56%
B) 32%
C) 18%
D) 44%
E) 75%
A) 56%
B) 32%
C) 18%
D) 44%
E) 75%

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
32
If a heat engine has an efficiency of 20% and its power output is 660 W,what is the rate of heat input from the combustion phase?
A) 132 W
B) 3,400 W
C) 3,300 W
D) 3,200 W
E) 3,100 W
A) 132 W
B) 3,400 W
C) 3,300 W
D) 3,200 W
E) 3,100 W

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
33
An electrical generating plant operates at a boiler temperature of 229°C and exhausts the unused heat into a nearby river at 18°C.If the generating plant has a power output of 870 megawatts (MW)and if the actual efficiency is 3/4 the theoretical efficiency,how much heat per second must be delivered to the boiler? (0°C = 273 K)
A) 8,279 MW
B) 4,140 MW
C) 2,070 MW
D) 2,760 MW
E) 1,600 MW
A) 8,279 MW
B) 4,140 MW
C) 2,070 MW
D) 2,760 MW
E) 1,600 MW

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
34
Suppose a power plant uses a Carnot engine to generate electricity,using the atmosphere at 300 K as the low-temperature reservoir.Suppose the power plant produces 1 × 106 J of electricity with the hot reservoir at 500 K during Day One and then produces 1 × 106 J of electricity with the hot reservoir at 600 K during Day Two.The thermal pollution was:
A) greatest on Day One.
B) greatest on Day Two.
C) the same on both days.
D) zero on both days.
A) greatest on Day One.
B) greatest on Day Two.
C) the same on both days.
D) zero on both days.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
35
A cylinder containing an ideal gas has a volume of 2 m3 and a pressure of 1.7 × 105 Pa at a temperature of 400 K.The cylinder is placed against a metal block that is maintained at 900 K and the gas expands as the pressure remains constant until the temperature of the gas reaches 900 K.The change in internal energy of the gas is +6.5 × 105 J.How much heat did the gas absorb?
A) 0
B) 4.6 × 105 J
C) 6.5 × 105 J
D) 10.8 × 105 J
E) 4.3 × 105 J
A) 0
B) 4.6 × 105 J
C) 6.5 × 105 J
D) 10.8 × 105 J
E) 4.3 × 105 J

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
36
Heat is applied to an ice-water mixture to melt some of the ice.In this process:
A) work is done by the ice-water mixture.
B) the temperature increases.
C) the internal energy increases.
D) all of the above are correct.
A) work is done by the ice-water mixture.
B) the temperature increases.
C) the internal energy increases.
D) all of the above are correct.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
37
A thermodynamic process that happens very quickly tends to be:
A) isobaric.
B) isothermal.
C) isovolumetric.
D) adiabatic.
A) isobaric.
B) isothermal.
C) isovolumetric.
D) adiabatic.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
38
The maximum theoretical thermodynamic efficiency of a heat engine operating between hot and cold reservoirs is a function of which of the following?
A) hot reservoir temperature only
B) cold reservoir temperature only
C) both hot and cold reservoir temperatures
D) None of the above choices are valid.
A) hot reservoir temperature only
B) cold reservoir temperature only
C) both hot and cold reservoir temperatures
D) None of the above choices are valid.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
39
How much thermal energy must be added to 7.9 moles of a diatomic ideal gas to raise its temperature 22.4 K?
A) 735 J
B) 2,206 J
C) 5,147 J
D) 3,676 J
E) 3,964 J
A) 735 J
B) 2,206 J
C) 5,147 J
D) 3,676 J
E) 3,964 J

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
40
During each cycle of operation a refrigerator absorbs 51 cal from the freezer compartment and expels 84 cal to the room.If one cycle occurs every 10 s,how many minutes will it take to freeze 550 g of water,initially at 0°C? (Lv = 80 cal/g)
A) 72 min
B) 1,438 min
C) 144 min
D) 87 min
E) 863 min
A) 72 min
B) 1,438 min
C) 144 min
D) 87 min
E) 863 min

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
41
On a P-V diagram,if a process involves a closed curve,the area inside the curve represents:
A) internal energy.
B) heat.
C) work.
D) zero.
A) internal energy.
B) heat.
C) work.
D) zero.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
42
Of the following heat engines,which has the highest efficiency?
A) Hero's engine
B) a Carnot engine
C) a car's gasoline engine
D) a truck's diesel engine
A) Hero's engine
B) a Carnot engine
C) a car's gasoline engine
D) a truck's diesel engine

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
43
A 1.0-kg chunk of ice at 0°C melts,absorbing 80 000 cal of heat in the process.Which of the following best describes what happens to this system?
A) increased entropy
B) lost entropy
C) entropy maintained constant
D) work converted to energy
A) increased entropy
B) lost entropy
C) entropy maintained constant
D) work converted to energy

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following choices is an appropriate unit for measuring entropy changes?
A) J⋅K
B) N⋅K
C) J/s
D) J/K
A) J⋅K
B) N⋅K
C) J/s
D) J/K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
45
The P-V diagram of a cyclic process shows a curve that encloses an area.The work done by the heat engine,represented by the enclosed area,is positive when the path around the area proceeds in which of the following fashions?
A) clockwise
B) counterclockwise
C) It is always positive.
D) It is always negative.
A) clockwise
B) counterclockwise
C) It is always positive.
D) It is always negative.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
46
1.2 kilogram of water at 1.00 atm at the boiling point of 100°C is heated until all the water vaporizes.What is its change in entropy? (For water,Lv = 2.26 × 106 J/kg)
A) 14,542 J/K
B) 7,271 J/K
C) 3,635 J/K
D) 2,424 J/K
E) 9,934 J/K
A) 14,542 J/K
B) 7,271 J/K
C) 3,635 J/K
D) 2,424 J/K
E) 9,934 J/K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
47
If one could observe the individual atoms making up a piece of matter and note that during a process of change their motion somehow became more orderly,then one may assume which of the following in regard to the system?
A) increases in entropy
B) decreases in entropy
C) gains in thermal energy
D) positive work done on
A) increases in entropy
B) decreases in entropy
C) gains in thermal energy
D) positive work done on

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
48
The surface of the Sun is at approximately 5 700 K and the temperature of the Earth's surface is about 290 K.What total entropy change occurs when 980 J of heat energy is transferred from the Sun to the Earth?
A) 3.38 J/K
B) 3.21 J/K
C) 0.17 J/K
D) 3.55 J/K
E) 5.38 J/K
A) 3.38 J/K
B) 3.21 J/K
C) 0.17 J/K
D) 3.55 J/K
E) 5.38 J/K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
49
The efficiency of a Carnot engine operating between 95°C and 0°C is most nearly:
A) 13%.
B) 52%.
C) 26%.
D) 80%.
E) 9%.
A) 13%.
B) 52%.
C) 26%.
D) 80%.
E) 9%.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
50
A cylinder containing an ideal gas has a volume of 2 m3 and a pressure of 1.6 × 105 Pa at a temperature of 320 K.The cylinder is placed against a metal block that is maintained at 840 K and the gas expands as the pressure remains constant until the temperature of the gas reaches 840 K.The change in internal energy of the gas is 6.7 × 105 J.Find the change in entropy of the block associated with the heat transfer to the gas.
A) 0
B) -798 J/K
C) 2,094 J/K
D) -1,288 J/K
E) -1,923 J/K
A) 0
B) -798 J/K
C) 2,094 J/K
D) -1,288 J/K
E) -1,923 J/K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
51
A refrigerator has a coefficient of performance of 4.When removing 2.3 × 104 J from inside the refrigerator,how much energy is sent into the environment?
A) 0.6 × 104 J
B) 2.9 × 104 J
C) 9.2 × 104 J
D) 11.5 × 104 J
E) 3.6 × 104 J
A) 0.6 × 104 J
B) 2.9 × 104 J
C) 9.2 × 104 J
D) 11.5 × 104 J
E) 3.6 × 104 J

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
52
An 870-MW electric power plant has an efficiency of 30%.It loses its waste heat in large cooling towers.Approximately how much waste heat (in MJ)is discharged to the atmosphere per second?
A) 3,770 MJ
B) 2,030 MJ
C) 870 MJ
D) 2,900 MJ
E) 4,060 MJ
A) 3,770 MJ
B) 2,030 MJ
C) 870 MJ
D) 2,900 MJ
E) 4,060 MJ

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
53
A gasoline engine with an efficiency of 33.8% operates between a high temperature T1 and a low temperature T2 = 370 K.If this engine operates with Carnot efficiency,what is the high-side temperature T1?
A) 1,095 K
B) 844 K
C) 1,275 K
D) 559 K
E) 245 K
A) 1,095 K
B) 844 K
C) 1,275 K
D) 559 K
E) 245 K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
54
A 2.05-kg block of ice is at STP (0°C,1 atm)while it melts completely to water.What is its change in entropy? (For ice,Lf = 3.34 × 105 J/kg)
A) zero
B) 1,254 J/K
C) 627 J/K
D) 2,508 J/K
E) 5,016 J/K
A) zero
B) 1,254 J/K
C) 627 J/K
D) 2,508 J/K
E) 5,016 J/K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
55
The Carnot cycle consists of a combination of ____ and ____ processes.
A) isobaric,isovolumetric
B) isovolumetric,adiabatic
C) isobaric,isothermal
D) adiabatic,isothermal
A) isobaric,isovolumetric
B) isovolumetric,adiabatic
C) isobaric,isothermal
D) adiabatic,isothermal

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
56
An avalanche of ice and snow of mass 2,000 kg slides a vertical distance of 180 m down a mountainside.If the temperature of the ice,snow,mountain and surrounding air are all at 0°C,what is the change in entropy of the universe?
A) 25,846 J/K
B) 12,923 J/K
C) 6,462 J/K
D) 4308 J/K
E) 1,319 J/K
A) 25,846 J/K
B) 12,923 J/K
C) 6,462 J/K
D) 4308 J/K
E) 1,319 J/K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
57
A Carnot engine runs between a hot reservoir at Th and a cold reservoir at Tc.If one of the temperatures is either increased or decreased by 3.5 K,which of the following changes would increase the efficiency by the greatest amount?
A) increasing Th
B) increasing Tc
C) decreasing Tc
D) cannot be determined from information given
A) increasing Th
B) increasing Tc
C) decreasing Tc
D) cannot be determined from information given

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following choices best corresponds to what is required by the second law of thermodynamics for any process taking place in an isolated system?
A) entropy decreases
B) entropy remains constant
C) entropy increases
D) entropy equals work done on the system
A) entropy decreases
B) entropy remains constant
C) entropy increases
D) entropy equals work done on the system

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
59
What is the change in entropy (ΔS)when one mole of silver (102 g)is completely melted at 962°C? (The heat of fusion of silver is 8.82 × 104 J/kg. )
A) 9.35 J/K
B) 7.28 J/K
C) 3.64 J/K
D) 14.57 J/K
E) 5.28 J/K
A) 9.35 J/K
B) 7.28 J/K
C) 3.64 J/K
D) 14.57 J/K
E) 5.28 J/K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
60
According to the first law of thermodynamics,for any process that may occur within an isolated system,which of the following choices applies?
A) entropy remains constant
B) entropy increases
C) entropy decreases
D) None of the above choices apply.
A) entropy remains constant
B) entropy increases
C) entropy decreases
D) None of the above choices apply.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
61
A pound of body fat has an energy content of about 4,100 kcal.If a 1,300-kg automobile had an equivalent amount of translational kinetic energy,how fast would it be moving? (0.447 m/s = 1 mph,1 kcal = 4 186 J)
A) 115 mph
B) 257 mph
C) 82 mph
D) 364 mph
E) 182 mph
A) 115 mph
B) 257 mph
C) 82 mph
D) 364 mph
E) 182 mph

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
62
On an average diet,the consumption of 11 liters of oxygen releases how much energy? (4.1 kcal are released per liter of oxygen consumed. )
A) 41 kJ
B) 189 kJ
C) 46 kJ
D) 4,600 kJ
E) 377 kJ
A) 41 kJ
B) 189 kJ
C) 46 kJ
D) 4,600 kJ
E) 377 kJ

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
63
Entropy is a measure of the ____ of a system.
A) disorder
B) temperature
C) heat
D) internal energy
A) disorder
B) temperature
C) heat
D) internal energy

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
64
At the dice factory,sets of novelty dice are created where a side with 7 dots replaces the single dot side.The sides then range from 2 to 7 instead of the usually 1 to 6.Using such dice for a game of craps,what will be the most probable roll?
A) 7,this change in dots results in the same most probable value.
B) 7.5
C) 8
D) 9
A) 7,this change in dots results in the same most probable value.
B) 7.5
C) 8
D) 9

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
65
When considering human metabolism in terms of the 1st Law of Thermodynamics,which of the following represents the metabolic rate?
A) ΔU / Δt
B) ΔQ / Δt
C) W / Δt
D) ΔW / Δt
A) ΔU / Δt
B) ΔQ / Δt
C) W / Δt
D) ΔW / Δt

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
66
On a PV diagram,2 curves are plotted,both starting at the same point and both ending at the same final increased volume.One curve is for an isothermal process;the other is for an adiabatic process.What does the area between these two curves represent?
A) Q absorbed by the isothermal process.
B) W done by the adiabatic process.
C) ΔU for the isothermal process.
D) Neither Q,W,nor ΔU for either of the processes is represented.
A) Q absorbed by the isothermal process.
B) W done by the adiabatic process.
C) ΔU for the isothermal process.
D) Neither Q,W,nor ΔU for either of the processes is represented.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
67
In an isovolumetric process where the pressure increases,are the heat absorbed,work done by the system,and the change in internal energy of the system positive,negative,or zero?
A) Q is +,W is +,and ΔU is +.
B) Q is +,W is −,and ΔU is 0.
C) Q is +,W is 0,and ΔU is +.
D) Q is −,W is 0,and ΔU is −.
A) Q is +,W is +,and ΔU is +.
B) Q is +,W is −,and ΔU is 0.
C) Q is +,W is 0,and ΔU is +.
D) Q is −,W is 0,and ΔU is −.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
68
On a PV diagram,2 curves are plotted,both starting at the same point (P1,V1)and both ending at the same increased volume (V2).One curve is for an isothermal process;the other is for an adiabatic process.Except for the common starting point,which curve is the upper one?
A) The isothermal process curve will always be the upper one.
B) The adiabatic process curve will always be the upper one.
C) Since they start at the same point and end at the same volume,they will coincide.
D) The isothermal one will start out higher,but the adiabatic one will eventually cross it.
A) The isothermal process curve will always be the upper one.
B) The adiabatic process curve will always be the upper one.
C) Since they start at the same point and end at the same volume,they will coincide.
D) The isothermal one will start out higher,but the adiabatic one will eventually cross it.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
69
Three Carnot engines operate between temperature reservoirs as follows: Engine A: Th = 1,100 K,Tc = 1,000 K;Engine B: Th = 1,100 K,Tc = 500 K;Engine C: Th = 550 K,Tc = 500 K.Which two engines have the same thermal efficiency?
A) A and B
B) B and C
C) A and C
D) No two have the same thermal efficiency.
E) A and B and C
A) A and B
B) B and C
C) A and C
D) No two have the same thermal efficiency.
E) A and B and C

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
70
A person consumes 2,100 kcal/day while expending 3,400 kcal/day.In a month's time,about how much weight would this person lose if the loss were essentially all from body fat? (Body fat has an energy content of about 4 100 kcal per pound. )
A) 19 pound
B) 29 pounds
C) 10 pounds
D) 5 pounds
E) 38 pounds
A) 19 pound
B) 29 pounds
C) 10 pounds
D) 5 pounds
E) 38 pounds

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck


