Exam 12: The Laws of Thermodynamics Work in Thermodynamic Processes
Exam 1: Introduction60 Questions
Exam 1: Introduction: Part A47 Questions
Exam 2: Motion in One Dimension64 Questions
Exam 2: Motion in One Dimension: Part A42 Questions
Exam 3: Vectors and Two-Dimensional Motion74 Questions
Exam 3: Vectors and Two-Dimensional Motion: Part A64 Questions
Exam 4: The Laws of Motion93 Questions
Exam 4: The Laws of Motion: Part A69 Questions
Exam 5: Energy84 Questions
Exam 5: Energy: Part A32 Questions
Exam 6: Momentum and Collisions83 Questions
Exam 6: Momentum and Collisions: Part A61 Questions
Exam 7: Rotational Motion and the Law of Gravity84 Questions
Exam 7: Rotational Motion and the Law of Gravity: Part A48 Questions
Exam 8: Rotational Equilibrium and Rotational Dynamics60 Questions
Exam 8: Rotational Equilibrium and Rotational Dynamics: Part A61 Questions
Exam 9: Solids and Fluids78 Questions
Exam 9: Solids and Fluids: Part A46 Questions
Exam 10: Thermal Physics82 Questions
Exam 10: Thermal Physics: Part A56 Questions
Exam 11: Energy in Thermal Processes Heat and Internal Energy82 Questions
Exam 11: Energy in Thermal Processes Heat and Internal Energy: Part A54 Questions
Exam 12: The Laws of Thermodynamics Work in Thermodynamic Processes70 Questions
Exam 12: The Laws of Thermodynamics Work in Thermodynamic Processes: Part A40 Questions
Exam 13: Vibrations and Waves83 Questions
Exam 13: Vibrations and Waves: Part A48 Questions
Exam 14: Sound81 Questions
Exam 14: Sound: Part A67 Questions
Exam 15: Electric Forces and Electric Fields81 Questions
Exam 15: Electric Forces and Electric Fields: Part A42 Questions
Exam 16: Electrical Energy and Capacitance81 Questions
Exam 16: Electrical Energy and Capacitance: Part A33 Questions
Exam 17: Current and Resistance Electric Current83 Questions
Exam 17: Current and Resistance Electric Current: Part A37 Questions
Exam 18: Direct-Current Circuits Sources of EMF77 Questions
Exam 18: Direct-Current Circuits Sources of EMF: Part A54 Questions
Exam 19: Magnetism Magnets82 Questions
Exam 19: Magnetism Magnets: Part A67 Questions
Exam 20: Induced Voltages and Inductance83 Questions
Exam 20: Induced Voltages and Inductance: Part A46 Questions
Exam 21: Alternating-Current Circuits and Electromagnetic Waves98 Questions
Exam 21: Alternating-Current Circuits and Electromagnetic Waves: Part A32 Questions
Exam 22: Reflection and Refraction of Light81 Questions
Exam 22: Reflection and Refraction of Light: Part A49 Questions
Exam 23: Mirrors and Lenses82 Questions
Exam 23: Mirrors and Lenses: Part A31 Questions
Exam 24: Wave Optics88 Questions
Exam 24: Wave Optics: Part A71 Questions
Exam 25: Optical Instruments79 Questions
Exam 25: Optical Instruments: Part A59 Questions
Exam 26: Relativity62 Questions
Exam 26: Relativity: Part A29 Questions
Exam 27: Quantum Physics Blackbody Radiation and Plancks Hypothesis79 Questions
Exam 27: Quantum Physics Blackbody Radiation and Plancks Hypothesis: Part A52 Questions
Exam 28: Atomic Physics71 Questions
Exam 28: Atomic Physics: Part A38 Questions
Exam 29: Nuclear Physics75 Questions
Exam 29: Nuclear Physics: Part A43 Questions
Exam 30: Nuclear Energy and Elementary Particles88 Questions
Exam 30: Nuclear Energy and Elementary Particles: Part A37 Questions
Exam 31: Particle Collisions, Mediating Photons, and Quark Structures: Exploring the Fundamentals of Physics16 Questions
Select questions type
Heat is applied to an ice-water mixture to melt some of the ice.In this process:
Free
(Multiple Choice)
5.0/5  (30)
(30)
Correct Answer:
C
At the dice factory,sets of novelty dice are created where a side with 7 dots replaces the single dot side.The sides then range from 2 to 7 instead of the usually 1 to 6.Using such dice for a game of craps,what will be the most probable roll?
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
D
In the first law of thermodynamics, 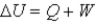 ,W is positive when
,W is positive when
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
B
The efficiency of a Carnot engine operating between 95°C and 0°C is most nearly:
(Multiple Choice)
5.0/5  (36)
(36)
If an ideal gas does positive work on its surroundings,we may assume,with regard to the gas:
(Multiple Choice)
4.8/5  (38)
(38)
System 1 is 3.6 moles of a monatomic ideal gas held at a constant volume as it undergoes a temperature increase.System 2 is 3.6 moles of a diatomic ideal gas held at constant volume as it also undergoes a temperature increase.The thermal energy absorbed by System 1 is 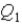 ,and the thermal energy absorbed by System 2 is
,and the thermal energy absorbed by System 2 is 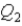 As both systems undergo a temperature increase of 74.6 K.What is the ratio
As both systems undergo a temperature increase of 74.6 K.What is the ratio 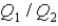 ?
?
(Multiple Choice)
4.8/5  (34)
(34)
According to the first law of thermodynamics,for any process that may occur within an isolated system,which of the following choices applies?
(Multiple Choice)
5.0/5  (29)
(29)
How much thermal energy must be added to 7.9 moles of a diatomic ideal gas to raise its temperature 22.4 K?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following choices best corresponds to what is required by the second law of thermodynamics for any process taking place in an isolated system?
(Multiple Choice)
4.8/5  (34)
(34)
On a PV diagram,2 curves are plotted,both starting at the same point and both ending at the same final increased volume.One curve is for an isothermal process;the other is for an adiabatic process.What does the area between these two curves represent?
(Multiple Choice)
5.0/5  (37)
(37)
A quantity of monatomic ideal gas expands adiabatically from a volume of 2.4 liters to 6.7 liters.If the initial pressure is P0,what is the final pressure?
(Multiple Choice)
4.8/5  (34)
(34)
In an isovolumetric process where the pressure increases,are the heat absorbed,work done by the system,and the change in internal energy of the system positive,negative,or zero?
(Multiple Choice)
4.8/5  (44)
(44)
A cylinder containing an ideal gas has a volume of 2 m3 and a pressure of 1.6 × 105 Pa at a temperature of 320 K.The cylinder is placed against a metal block that is maintained at 840 K and the gas expands as the pressure remains constant until the temperature of the gas reaches 840 K.The change in internal energy of the gas is 6.7 × 105 J.Find the change in entropy of the block associated with the heat transfer to the gas.
(Multiple Choice)
5.0/5  (45)
(45)
When gasoline is burned,it gives off 47,000 J/g of heat energy.If an automobile uses 12.7 kg of gasoline per hour with an efficiency of 24%,what is the average horsepower output of the engine? (1 hp = 746 W)
(Multiple Choice)
4.7/5  (29)
(29)
A person consumes 2,100 kcal/day while expending 3,400 kcal/day.In a month's time,about how much weight would this person lose if the loss were essentially all from body fat? (Body fat has an energy content of about 4 100 kcal per pound. )
(Multiple Choice)
4.8/5  (36)
(36)
If a heat engine has an efficiency of 20% and its power output is 660 W,what is the rate of heat input from the combustion phase?
(Multiple Choice)
4.7/5  (37)
(37)
On an average diet,the consumption of 11 liters of oxygen releases how much energy? (4.1 kcal are released per liter of oxygen consumed. )
(Multiple Choice)
4.9/5  (39)
(39)
A 2.0-mol ideal gas system is maintained at a constant volume of 4.0 L.If 100 J of heat is added,what is the work done on the system?
(Multiple Choice)
4.7/5  (44)
(44)
Of the following heat engines,which has the highest efficiency?
(Multiple Choice)
4.8/5  (30)
(30)
Suppose a power plant uses a Carnot engine to generate electricity,using the atmosphere at 300 K as the low-temperature reservoir.Suppose the power plant produces 1 × 106 J of electricity with the hot reservoir at 500 K during Day One and then produces 1 × 106 J of electricity with the hot reservoir at 600 K during Day Two.The thermal pollution was:
(Multiple Choice)
4.7/5  (34)
(34)
Showing 1 - 20 of 70
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)