Deck 7: Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/82
Play
Full screen (f)
Deck 7: Acids and Bases
1
The following represents the structure of a hydronium ion. 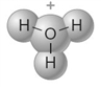

True
2
The pKa of lactic acid is 3.86, an equimolar solution of lactic acid and potassium lactate will have a pH of 7.72.
False
3
The following is an accurate description of the ionization of HBr in water. 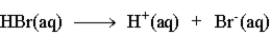
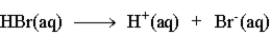
False
4
A solution is 0.10 M in ascorbic acid (Vitamin C), H2C6H6O6, and 0.10 M in sodium ascorbate, NaHC6H6O6. When base is added to the solution, H2C6H6O6 neutralizes the added base.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
5
Consider the following reaction.
CH3OH(l) + CH3OH(l)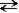 CH3O-(l) + CH3OH2+(l)
CH3O-(l) + CH3OH2+(l)
This reaction could be classified as both an autoionization reaction and a proton transfer reaction.
CH3OH(l) + CH3OH(l)
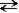 CH3O-(l) + CH3OH2+(l)
CH3O-(l) + CH3OH2+(l)This reaction could be classified as both an autoionization reaction and a proton transfer reaction.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
6
A solution composed of HCl and NaCl would produce an effective buffer.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
7
An aqueous solution contains a [H3O+] that is 1.0 × 10-3 M. What is the [OH-] of this solution?
A)1.0 × 10-14 M
B)1.0 × 10-11 M
C)1.0 × 10-3 M
D)1.0 × 1011 M
A)1.0 × 10-14 M
B)1.0 × 10-11 M
C)1.0 × 10-3 M
D)1.0 × 1011 M

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
8
For a healthy individual, the pH of the blood in the veins is lower than in the arteries.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is present in pure water?
A)H3O+
B)OH−
C)H2O
D)All are present in pure water.
A)H3O+
B)OH−
C)H2O
D)All are present in pure water.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
10
The range of breaths per minute, also known as respiratory rate, for a normal, healthy adult is 12-20 breaths per minute. A breathing rate of higher than this is termed hyperventilation.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
11
The following solutions are ranked from the most acidic to the most basic.
Most acidic urine (pH 5.89) intestine contents (pH 8.06) blood (pH 7.30) Most basic
Most acidic urine (pH 5.89) intestine contents (pH 8.06) blood (pH 7.30) Most basic

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
12
Adipic acid has the formula given below. 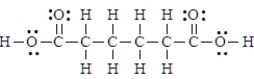 This is a polyprotic acid.
This is a polyprotic acid.
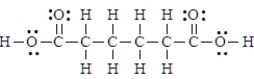 This is a polyprotic acid.
This is a polyprotic acid.
Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
13
The molar concentration of H3O+ in an aqueous solution would be increased by the addition of KOH.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
14
Both the kidneys and the carbon dioxide-carbonic acid cycle help regulate blood pH.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
15
In converting from the pH of a solution to the corresponding H3O+ concentration, the following equation should be used.
[H3O+] = 10-pH
[H3O+] = 10-pH

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
16
Below is the structure for the amino acid alanine. 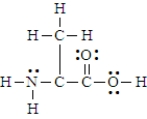 Alanine can undergo an internal acid-base reaction.
Alanine can undergo an internal acid-base reaction. 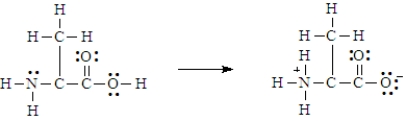 In this reaction, the -NH2 group functions as a base.
In this reaction, the -NH2 group functions as a base.
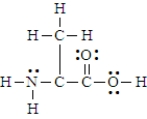 Alanine can undergo an internal acid-base reaction.
Alanine can undergo an internal acid-base reaction.  In this reaction, the -NH2 group functions as a base.
In this reaction, the -NH2 group functions as a base.
Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
17
Nitroaniline (C6H6N2O2) is a weak base. This would indicate that the pH of a 0.010 M solution of nitroaniline will be less than 7.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
18
Very rapid breathing can lead to a condition know as respiratory acidosis.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
19
H+, H3O+, and OH+ are different ways of representing the species that causes a solution to be acidic.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
20
Adipic acid has the formula given below. 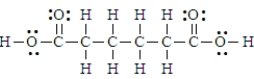 Thus substance would be classified as amphiprotic.
Thus substance would be classified as amphiprotic.
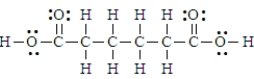 Thus substance would be classified as amphiprotic.
Thus substance would be classified as amphiprotic.
Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is the conjugate acid of ammonia, NH3?
A)H+
B)H3O+
C)NH2-
D)NH4+
A)H+
B)H3O+
C)NH2-
D)NH4+

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following solutions can function as a buffer?
A)a solution containing HC2H3O2 and NaC2H3O2
B)a solution containing H2CO3 and NaHCO3
C)a solution containing NH3 and NH4Cl
D)all of the above
A)a solution containing HC2H3O2 and NaC2H3O2
B)a solution containing H2CO3 and NaHCO3
C)a solution containing NH3 and NH4Cl
D)all of the above

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
23
Ascorbic acid (Vitamin C), H2C6H6O6, has a pKa of 4.10. A solution is 0.10 M in ascorbic acid and 0.20 M in sodium ascorbate, NaHC6H6O6. What is the approximate pH of the solution?
A)4.10
B)slightly less than 4.10
C)slightly more than 4.10
D)The solution will have a pH of exactly 7.
A)4.10
B)slightly less than 4.10
C)slightly more than 4.10
D)The solution will have a pH of exactly 7.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
24
When a solution of HF (a weak acid) is added to a solution containing HPO42- (here, a base), the equation for reaction that occurs is:
A)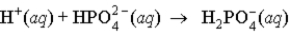
B)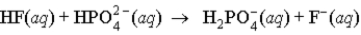
C)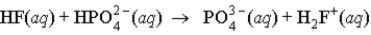
D)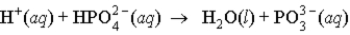
A)
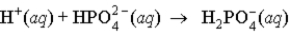
B)
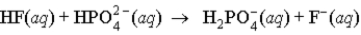
C)
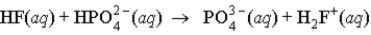
D)
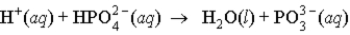

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
25
An ammonia solution has a pH of 11.30, what is the H3O+ concentration in this solution?
A)5.0 × 10-23 M
B)2.0 × 10-9 M
C)5.0 × 10-12 M
D)2.0 × 1011 M
A)5.0 × 10-23 M
B)2.0 × 10-9 M
C)5.0 × 10-12 M
D)2.0 × 1011 M

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
26
Consider the following reaction.
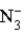 + H2O
+ H2O 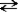 HN3 + OH−
HN3 + OH−
This reaction indicates that:
A)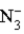 is a weak base.
is a weak base.
B)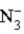 is a strong base.
is a strong base.
C)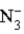 is a weak acid.
is a weak acid.
D)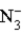 is a strong acid.
is a strong acid.
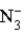 + H2O
+ H2O 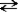 HN3 + OH−
HN3 + OH− This reaction indicates that:
A)
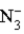 is a weak base.
is a weak base.B)
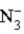 is a strong base.
is a strong base.C)
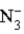 is a weak acid.
is a weak acid.D)
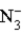 is a strong acid.
is a strong acid.
Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following pH's corresponds to a highly acidic solution?
A)2.1
B)6.8
C)7.2
D)11.2
A)2.1
B)6.8
C)7.2
D)11.2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
28
When fumaric acid, H2C4H2O4 reacts with NaOH, the second reaction that occurs is
A)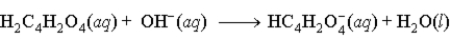
B)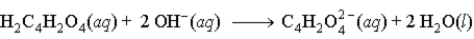
C)
D)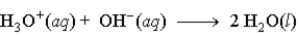
A)
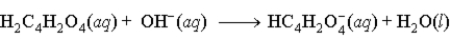
B)
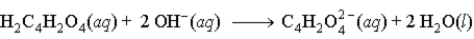
C)

D)
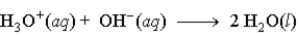

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
29
A sodium hydroxide solution has a pH of 12.40, what is the OH- concentration in this solution?
A)4.0 × 101 M
B)4.0 × 10-13 M
C)2.5 × 10- 2 M
D)2.5 × 1012 M
A)4.0 × 101 M
B)4.0 × 10-13 M
C)2.5 × 10- 2 M
D)2.5 × 1012 M

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is the conjugate base of H3PO4?
A)H2PO4-
B)HPO42-
C)PO43-
D)OH-
E)both H2PO4- and HPO42-
A)H2PO4-
B)HPO42-
C)PO43-
D)OH-
E)both H2PO4- and HPO42-

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
31
Oxalic acid (H2C2O4) is weak acid found in the leaves of rhubarb. Which of the following solutions will have the highest pH?
A)1.0 M H2C2O4
B)0.5 M H2C2O4
C)1.0 × 10-4 M H2C2O4
D)0.00075 M H2C2O4
A)1.0 M H2C2O4
B)0.5 M H2C2O4
C)1.0 × 10-4 M H2C2O4
D)0.00075 M H2C2O4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following substances is amphiprotic?
A)HCO3-
B)HPO43-
C)H2PO4-
D)All are amphiprotic.
A)HCO3-
B)HPO43-
C)H2PO4-
D)All are amphiprotic.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
33
In an aqueous solution. the [OH-] is 2.0 × 10-3 M. What is the [H3O+] of this solution?
A)5.0 × 10-12 M
B)2.0 × 1011 M
C)2.0 × 10-3 M
D)5.0 × 1012 M
A)5.0 × 10-12 M
B)2.0 × 1011 M
C)2.0 × 10-3 M
D)5.0 × 1012 M

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following statements best describes the relationship between conjugate acids and bases?
A)As acid strength increases, the conjugate base strength increases.
B)As base strength increases, the conjugate acid strength decreases.
C)The conjugate base of a strong acid is always strong.
D)There is no specific correlation.
A)As acid strength increases, the conjugate base strength increases.
B)As base strength increases, the conjugate acid strength decreases.
C)The conjugate base of a strong acid is always strong.
D)There is no specific correlation.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
35
A buffer is composed of ammonia (NH3) and ammonium chloride (NH4Cl). When base is added to the buffer, which of the following reactions occurs?
A)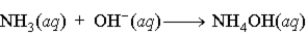
B)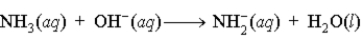
C)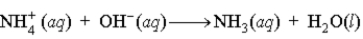
D)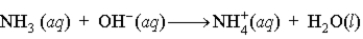
A)
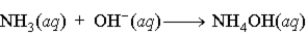
B)
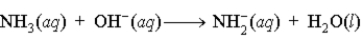
C)
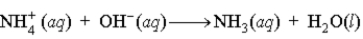
D)
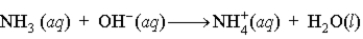

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is a polyprotic acid?
A)HCl
B)HNO3
C)H2SO4
D)H3PO4
E)both c and d
A)HCl
B)HNO3
C)H2SO4
D)H3PO4
E)both c and d

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
37
The chemical equation for the reaction of the base, ethoxide, CH3CH2O-, with water would show the products:
A)CH3CH2OH and OH-
B)CH3CH3 and H3O+
C)CH3CH2OH and H3O+
D)CH3CH3 and OH-
A)CH3CH2OH and OH-
B)CH3CH3 and H3O+
C)CH3CH2OH and H3O+
D)CH3CH3 and OH-

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is characteristic of a buffer?
A)The pH will go down significantly when H3O+ is added to the buffer.
B)The pH will go down very slightly when H3O+ is added to a buffer.
C)The pH will go up significantly when H3O+ is added to the buffer.
D)The pH will go up very slightly when H3O+ is added to the buffer.
A)The pH will go down significantly when H3O+ is added to the buffer.
B)The pH will go down very slightly when H3O+ is added to a buffer.
C)The pH will go up significantly when H3O+ is added to the buffer.
D)The pH will go up very slightly when H3O+ is added to the buffer.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
39
All of the following solutions are 0.025 M, which of the solutions contains the highest concentration of OH-?
A)acetic acid (pH 3.17)
B)ascorbic acid (pH 6.70)
C)phenol (pH 5.73)
D)iodic acid (pH 1.60)
A)acetic acid (pH 3.17)
B)ascorbic acid (pH 6.70)
C)phenol (pH 5.73)
D)iodic acid (pH 1.60)

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
40
A water solution is found to have an OH- concentration of 6.3 × 10−10. The solution would be classified as:
A)acidic.
B)basic.
C)neutral.
A)acidic.
B)basic.
C)neutral.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
41
Consider the image which depicts the reaction between CH3COOH and H2O. Reactants:
Products:

If the composition of the equilibrium mixture is as represented below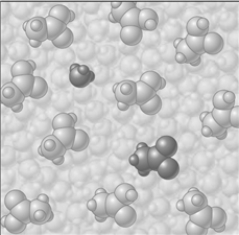
CH3COOH is classified as
A)strong electrolyte
B)weak electrolyte
C)nonelectrolyte
Products:

If the composition of the equilibrium mixture is as represented below

CH3COOH is classified as
A)strong electrolyte
B)weak electrolyte
C)nonelectrolyte

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following substances can react with both HCl and NaOH?.
A)Ca(HCO3)2
B)KCl
C)CH3CH2NH2
D)H2C2O4
A)Ca(HCO3)2
B)KCl
C)CH3CH2NH2
D)H2C2O4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
43
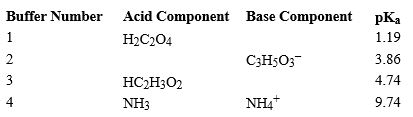 Using the above table,fill in the blank with the appropriate integer (1 ,2,3,...)indicating the buffer and/or the letter indicating the formula below.
Using the above table,fill in the blank with the appropriate integer (1 ,2,3,...)indicating the buffer and/or the letter indicating the formula below.In Buffer 1, the missing base component is _________________________.
A)H3C2O4+
B)HC2O4-
C)C3H4O32-
D)HC3H5O3
E)C2H3O2-
F)H2C2H3O2
G)C3H5O3-

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
44
 Using the above table,fill in the blank with the appropriate integer (1 ,2,3,...)indicating the buffer and/or the letter indicating the formula below.
Using the above table,fill in the blank with the appropriate integer (1 ,2,3,...)indicating the buffer and/or the letter indicating the formula below.Buffer ______________________ would be the most effective buffer in basic solutions.
A)H3C2O4+
B)HC2O4-
C)C3H4O32-
D)HC3H5O3
E)C2H3O2-
F)H2C2H3O2
G)C3H5O3-

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
45
Human blood contains which of the following buffers?
A)protein-H+/protein
B)H2PO4-/HPO42-
C)H2CO3/HCO3-
D)All of the above are involved.
A)protein-H+/protein
B)H2PO4-/HPO42-
C)H2CO3/HCO3-
D)All of the above are involved.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following is true of a buffer prepared with equal concentrations of an acid and its conjugate base?
A)pH = 7
B)pH = pKa
C)The pH depends on the concentration of the buffer.
D)none of the above
A)pH = 7
B)pH = pKa
C)The pH depends on the concentration of the buffer.
D)none of the above

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
47
 Using the above table,fill in the blank with the appropriate integer (1 ,2,3,...)indicating the buffer and/or the letter indicating the formula below.
Using the above table,fill in the blank with the appropriate integer (1 ,2,3,...)indicating the buffer and/or the letter indicating the formula below.In Buffer 2,_________________________ would react with added acid.
A)H3C2O4+
B)HC2O4-
C)C3H4O32-
D)HC3H5O3
E)C2H3O2-
F)H2C2H3O2
G)C3H5O3-

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is excreted by the kidneys to regulate the effect of excess protein in the diet?
A)NH4+
B)H3O+
C)HCO3-
D)H2PO4-
A)NH4+
B)H3O+
C)HCO3-
D)H2PO4-

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is a cause of respiratory acidosis?
A)a poor diet
B)hyperventilation
C)hypoventilation
D)intense exercise
A)a poor diet
B)hyperventilation
C)hypoventilation
D)intense exercise

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
50
When carbonated beverages are bottled or canned, the partial pressure of carbon dioxide above this liquid is maintained at a high pressure. This procedure cause the pH of the beverage to
A)increase.
B)decrease.
C)remain constant.
A)increase.
B)decrease.
C)remain constant.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following solutions is the most alkaline?
A)coffee (pH 5.12)
B)urine (pH 7.24)
C)dishwasher detergent (pH 2.38)
D)drain cleaner (pH 10.24)
A)coffee (pH 5.12)
B)urine (pH 7.24)
C)dishwasher detergent (pH 2.38)
D)drain cleaner (pH 10.24)

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
52
Consider the image which depicts the reaction between CH3COOH and H2O. Reactants:
Products:

If the composition of the equilibrium mixture is as represented below
A solution of KF in water will be
A)acidic.
B)basic.
C)neutral.
Products:

If the composition of the equilibrium mixture is as represented below

A solution of KF in water will be
A)acidic.
B)basic.
C)neutral.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
53
When NaOH reacts with HCl, the net ionic equation for the reaction is:
A)H+ + OH- → H2O
B)Na+ + Cl- → NaCl
C)NaOH + HCl → NaCl + H2O
D)NaOH → Na+ + OH-
E)HCl → H+ + Cl-
A)H+ + OH- → H2O
B)Na+ + Cl- → NaCl
C)NaOH + HCl → NaCl + H2O
D)NaOH → Na+ + OH-
E)HCl → H+ + Cl-

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following is most likely to be a base?
A)NaCl
B)CH4
C)CH3NH2
D)H2C2O4
A)NaCl
B)CH4
C)CH3NH2
D)H2C2O4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
55
Consider the image which depicts the reaction between CH3COOH and H2O. Reactants:
Products:

If the composition of the equilibrium mixture is as represented below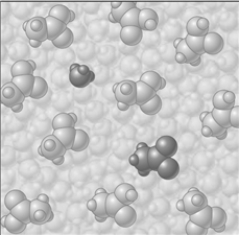
CH3COOH is classified as
A)strong acid
B)weak acid
C)strong base
D)weak base
Products:

If the composition of the equilibrium mixture is as represented below

CH3COOH is classified as
A)strong acid
B)weak acid
C)strong base
D)weak base

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following is most likely to be a salt?
A)Ca(NO3)2
B)CH3Cl
C)CH3CH2NH2
D)H2C2O4
A)Ca(NO3)2
B)CH3Cl
C)CH3CH2NH2
D)H2C2O4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
57
Metabolic acidosis resulting from vigorous exercise is associated with increased production of which of the following?
A)citric acid
B)formic acid
C)lactic acid
D)phosphoric acid
A)citric acid
B)formic acid
C)lactic acid
D)phosphoric acid

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following will determine the pH of a buffer made using acetic acid (HC2H3O2) and sodium acetate (NaC2H3O2)?
A)the amount of water present
B)the concentration of C2H3O2-
C)the concentration of HC2H3O2
D)the ratio of the concentrations of C2H3O2- and HC2H3O2
A)the amount of water present
B)the concentration of C2H3O2-
C)the concentration of HC2H3O2
D)the ratio of the concentrations of C2H3O2- and HC2H3O2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
59
Excess phosphorus is excreted by the kidneys. What effect does this have on the pH of blood plasma?
A)causes an increase
B)causes a decrease
C)has no effect
A)causes an increase
B)causes a decrease
C)has no effect

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
60
 Using the above table,fill in the blank with the appropriate integer (1 ,2,3,...)indicating the buffer and/or the letter indicating the formula below.
Using the above table,fill in the blank with the appropriate integer (1 ,2,3,...)indicating the buffer and/or the letter indicating the formula below.In Buffer 2, the missing acid component is_______________________.
A)H3C2O4+
B)HC2O4-
C)C3H4O32-
D)HC3H5O3
E)C2H3O2-
F)H2C2H3O2
G)C3H5O3-

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
61
Write the equation for the ionization of the weak acid, formic acid, HCHO2, in water.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
62
The buffer solution in the flask was prepared using one of the substances shown below.
citric acid (pKa 3.08)
NaH2PO4 (pKa 7.21)
NH4Cl (pKa 9.25)
Using the pH meter and solution shown in the image below, complete the following statements using one of the terms below.
complete the following statements using one of the terms below.
increase
decrease
remain constant
citric acid
NaH2PO4
NH4Cl
The buffer solution in the beaker could be prepared using__________.
citric acid (pKa 3.08)
NaH2PO4 (pKa 7.21)
NH4Cl (pKa 9.25)
Using the pH meter and solution shown in the image below,
 complete the following statements using one of the terms below.
complete the following statements using one of the terms below.increase
decrease
remain constant
citric acid
NaH2PO4
NH4Cl
The buffer solution in the beaker could be prepared using__________.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
63
The buffer solution in the flask was prepared using one of the substances shown below.
citric acid (pKa 3.08)
NaH2PO4 (pKa 7.21)
NH4Cl (pKa 9.25)
Using the pH meter and solution shown in the image below, complete the following statements using one of the terms below.
complete the following statements using one of the terms below.
increase
decrease
remain constant
citric acid
NaH2PO4
NH4Cl
If HNO were added to the buffer solution the pH of the solution would __________.
citric acid (pKa 3.08)
NaH2PO4 (pKa 7.21)
NH4Cl (pKa 9.25)
Using the pH meter and solution shown in the image below,
 complete the following statements using one of the terms below.
complete the following statements using one of the terms below.increase
decrease
remain constant
citric acid
NaH2PO4
NH4Cl
If HNO were added to the buffer solution the pH of the solution would __________.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
64
Consider the following three buffer reactions.
Buffer 1: protein-H+ and protein
Buffer 2: H2CO3 and HCO3-
Buffer 3: H2PO4- and HPO42-
Fill in the blank with the appropriate integer (1,2,or 3)to indicate the buffer system described.
Buffer ______________________is involved in preventing blood pH fluctuations due to hypo- and hyperventilation.
Buffer 1: protein-H+ and protein
Buffer 2: H2CO3 and HCO3-
Buffer 3: H2PO4- and HPO42-
Fill in the blank with the appropriate integer (1,2,or 3)to indicate the buffer system described.
Buffer ______________________is involved in preventing blood pH fluctuations due to hypo- and hyperventilation.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
65
If you dissolve 0.10 mol of a generic acid HA in 1.0 L of solution, the pH is 3.5. Is HA a strong or weak acid? Explain your answer.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
66
Consider the following three buffer reactions.
Buffer 1: protein-H+ and protein
Buffer 2: H2CO3 and HCO3-
Buffer 3: H2PO4- and HPO42-
Fill in the blank with the appropriate integer (1,2,or 3)to indicate the buffer system described.
Buffer 1 works in conjunction with buffer ____________________to regulate the pH of intracellular fluid.
Buffer 1: protein-H+ and protein
Buffer 2: H2CO3 and HCO3-
Buffer 3: H2PO4- and HPO42-
Fill in the blank with the appropriate integer (1,2,or 3)to indicate the buffer system described.
Buffer 1 works in conjunction with buffer ____________________to regulate the pH of intracellular fluid.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
67
Arterial blood gases (ABGs) were drawn on your patient. The following are the some of the results:
Respiratory Rate (RR) 10 breaths/min; pH 7.3; and Pressure of CO2 58 torr. The following questions are a basic step-by-step guide in evaluating ABGs.
a. If the normal range is 12 - 24 breaths/min , is this patient breathing within a normal range, hyperventilating, or hypoventilating?
b. Is this pH level on the acidic or basic side of the normal range?
c. Is this pressure of CO2 level within normal range, high, or low?
d. With the above findings, what is this patient experiencing?
Respiratory Rate (RR) 10 breaths/min; pH 7.3; and Pressure of CO2 58 torr. The following questions are a basic step-by-step guide in evaluating ABGs.
a. If the normal range is 12 - 24 breaths/min , is this patient breathing within a normal range, hyperventilating, or hypoventilating?
b. Is this pH level on the acidic or basic side of the normal range?
c. Is this pressure of CO2 level within normal range, high, or low?
d. With the above findings, what is this patient experiencing?

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
68
Joshua has had high blood pressure for a number of years. At Joshua's last checkup, his doctor orders tests to measure the amount of creatinine in Joshua's blood and urine. Creatinine is a compound that forms whenever muscles break down creatine, a chemical that helps supply energy for muscle contractions.
A) Does everyone have creatine in their circulating blood?
B) Does everyone secrete creatinine in their urine? Why or why not?
A) Does everyone have creatine in their circulating blood?
B) Does everyone secrete creatinine in their urine? Why or why not?

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
69
Consider the following three buffer reactions.
Buffer 1: protein-H+ and protein
Buffer 2: H2CO3 and HCO3-
Buffer 3: H2PO4- and HPO42-
Fill in the blank with the appropriate integer (1,2,or 3)to indicate the buffer system described.
Buffer______________________can have a variable pKa value.
Buffer 1: protein-H+ and protein
Buffer 2: H2CO3 and HCO3-
Buffer 3: H2PO4- and HPO42-
Fill in the blank with the appropriate integer (1,2,or 3)to indicate the buffer system described.
Buffer______________________can have a variable pKa value.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
70
The following reaction represents the self-ionization of ammonia (NH3).
NH3(aq)+ NH3(aq)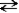 NH2-(aq)+ NH4+(aq)
NH2-(aq)+ NH4+(aq)
amide ammonium
Fill in the blanks with the appropriate terms from those listed below.
ammonia
ammonium
amide
In the reverse reaction, __________________________ion functions as an acid.
NH3(aq)+ NH3(aq)
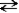 NH2-(aq)+ NH4+(aq)
NH2-(aq)+ NH4+(aq)amide ammonium
Fill in the blanks with the appropriate terms from those listed below.
ammonia
ammonium
amide
In the reverse reaction, __________________________ion functions as an acid.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
71
Consider the following three buffer reactions.
Buffer 1: protein-H+ and protein
Buffer 2: H2CO3 and HCO3-
Buffer 3: H2PO4- and HPO42-
Fill in the blank with the appropriate integer (1,2,or 3)to indicate the buffer system described.
Buffer______________________ is the most effective regulator for a pH of 7.2.
Buffer 1: protein-H+ and protein
Buffer 2: H2CO3 and HCO3-
Buffer 3: H2PO4- and HPO42-
Fill in the blank with the appropriate integer (1,2,or 3)to indicate the buffer system described.
Buffer______________________ is the most effective regulator for a pH of 7.2.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
72
What is the pH of a solution prepared by dissolving 0.400 g of HCl in enough water to produce 300.0 mL of solution?

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
73
Examine the structure given below 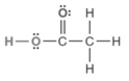 How many acidic hydrogen atoms are present? Enter a numerical value (1, 2, 3, ...).
How many acidic hydrogen atoms are present? Enter a numerical value (1, 2, 3, ...).
 How many acidic hydrogen atoms are present? Enter a numerical value (1, 2, 3, ...).
How many acidic hydrogen atoms are present? Enter a numerical value (1, 2, 3, ...).
Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
74
The buffer solution in the flask was prepared using one of the substances shown below.
citric acid (pKa 3.08)
NaH2PO4 (pKa 7.21)
NH4Cl (pKa 9.25)
Using the pH meter and solution shown in the image below, complete the following statements using one of the terms below.
complete the following statements using one of the terms below.
increase
decrease
remain constant
citric acid
NaH2PO4
NH4Cl
If KOH were added to the buffer solution the pH of the solution would __________.
citric acid (pKa 3.08)
NaH2PO4 (pKa 7.21)
NH4Cl (pKa 9.25)
Using the pH meter and solution shown in the image below,
 complete the following statements using one of the terms below.
complete the following statements using one of the terms below.increase
decrease
remain constant
citric acid
NaH2PO4
NH4Cl
If KOH were added to the buffer solution the pH of the solution would __________.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
75
In a solution of phosphoric acid all of the following species are present. Which is the least abundant?
PO43-
H2PO4-
HPO42-
PO43-
H2PO4-
HPO42-

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
76
If a patient were in diabetic ketoacidosis (DKA), would you expect their pH level to be high or low?

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
77
The following reaction represents the self-ionization of ammonia (NH3).
NH3(aq)+ NH3(aq)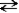 NH2-(aq)+ NH4+(aq)
NH2-(aq)+ NH4+(aq)
amide ammonium
Fill in the blanks with the appropriate terms from those listed below.
ammonia
ammonium
amide
In this reaction, the conjugate base of ammonia is the _______________________ ion.
NH3(aq)+ NH3(aq)
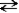 NH2-(aq)+ NH4+(aq)
NH2-(aq)+ NH4+(aq)amide ammonium
Fill in the blanks with the appropriate terms from those listed below.
ammonia
ammonium
amide
In this reaction, the conjugate base of ammonia is the _______________________ ion.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
78
Consider the following three buffer reactions.
Buffer 1: protein-H+ and protein
Buffer 2: H2CO3 and HCO3-
Buffer 3: H2PO4- and HPO42-
Fill in the blank with the appropriate integer (1,2,or 3)to indicate the buffer system described.
Buffer__________________is found in both plasma and red blood cells.
Buffer 1: protein-H+ and protein
Buffer 2: H2CO3 and HCO3-
Buffer 3: H2PO4- and HPO42-
Fill in the blank with the appropriate integer (1,2,or 3)to indicate the buffer system described.
Buffer__________________is found in both plasma and red blood cells.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
79
Write the equation for the ionization of weak base hydrazine, N2H4, in water.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
80
What is the pH of a 1 × 10-1 M solution of CaCl? Enter a numerical value.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck


