Exam 7: Acids and Bases
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds76 Questions
Exam 3: Chemical Bonds82 Questions
Exam 4: Energy and Physical Properties73 Questions
Exam 5: Solution Concentration76 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases82 Questions
Exam 8: Nuclear Chemistry65 Questions
Exam 9: Hydrocarbons: An Introduction to Organic Molecules72 Questions
Exam 10: Hydration, Dehydration, and Alcohols59 Questions
Exam 11: Carbonyl Compounds and Redox Reactions70 Questions
Exam 12: Organic Acids and Bases62 Questions
Exam 13: Condensation and Hydrolysis Reactions70 Questions
Exam 14: Proteins64 Questions
Exam 15: Carbohydrates73 Questions
Exam 16: Lipids and Membranes75 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity69 Questions
Select questions type
For a healthy individual, the pH of the blood in the veins is lower than in the arteries.
Free
(True/False)
4.9/5  (33)
(33)
Correct Answer:
False
Consider the following three buffer reactions.
Buffer 1: protein-H+ and protein
Buffer 2: H2CO3 and HCO3-
Buffer 3: H2PO4- and HPO42-
Fill in the blank with the appropriate integer (1,2,or 3)to indicate the buffer system described.
-Buffer ______________________is involved in preventing blood pH fluctuations due to hypo- and hyperventilation.
Free
(Short Answer)
4.8/5  (38)
(38)
Correct Answer:
2
Very rapid breathing can lead to a condition know as respiratory acidosis.
Free
(True/False)
4.8/5  (43)
(43)
Correct Answer:
False
The range of breaths per minute, also known as respiratory rate, for a normal, healthy adult is 12-20 breaths per minute. A breathing rate of higher than this is termed hyperventilation.
(True/False)
4.8/5  (34)
(34)
What is the pH of a solution prepared by dissolving 0.400 g of HCl in enough water to produce 300.0 mL of solution?
(Short Answer)
4.9/5  (39)
(39)
In a solution of phosphoric acid all of the following species are present. Which is the least abundant?
PO43-
H2PO4-
HPO42-
(Short Answer)
4.9/5  (37)
(37)
The following is an accurate description of the ionization of HBr in water. 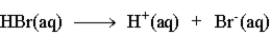
(True/False)
4.9/5  (29)
(29)
Write the equation for the ionization of weak base hydrazine, N2H4, in water.
(Essay)
4.9/5  (37)
(37)
What is the pH of a solution prepared by dissolving 4.00 g of NaOH in enough water to produce 500.0 mL of solution?
(Short Answer)
4.8/5  (27)
(27)
H+, H3O+, and OH+ are different ways of representing the species that causes a solution to be acidic.
(True/False)
4.9/5  (31)
(31)
Ascorbic acid (Vitamin C), H2C6H6O6, has a pKa of 4.10. A solution is 0.10 M in ascorbic acid and 0.20 M in sodium ascorbate, NaHC6H6O6. What is the approximate pH of the solution?
(Multiple Choice)
4.8/5  (33)
(33)
A sodium hydroxide solution has a pH of 12.40, what is the OH- concentration in this solution?
(Multiple Choice)
4.8/5  (36)
(36)
Write the equation for the ionization of the weak acid, formic acid, HCHO2, in water.
(Essay)
4.9/5  (32)
(32)
Consider the image which depicts the reaction between CH3COOH and H2O. Reactants:
Products:
 If the composition of the equilibrium mixture is as represented below
If the composition of the equilibrium mixture is as represented below 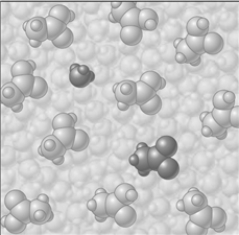 -CH3COOH is classified as
-CH3COOH is classified as
(Multiple Choice)
4.8/5  (32)
(32)
Consider the following three buffer reactions.
Buffer 1: protein-H+ and protein
Buffer 2: H2CO3 and HCO3-
Buffer 3: H2PO4- and HPO42-
Fill in the blank with the appropriate integer (1,2,or 3)to indicate the buffer system described.
-Buffer 1 works in conjunction with buffer ____________________to regulate the pH of intracellular fluid.
(Short Answer)
4.8/5  (41)
(41)
Showing 1 - 20 of 82
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)