Deck 1: Structure and Bonding
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/30
Play
Full screen (f)
Deck 1: Structure and Bonding
1
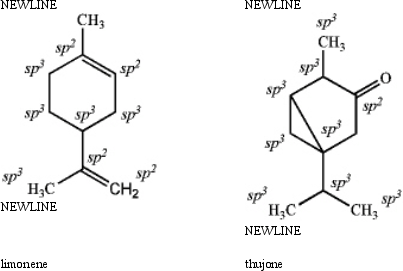
Specify the hybridization of each carbon atom of limonene,a natural product present in citrus fruits,and thujone,which is derived from wormwood,a traditional component of the notorious liquor,Absinthe.

2
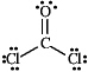
Draw all the lone pairs (nonbonding valence electrons)on the structure of phosgene,a poisonous gas once used as a chemical warfare agent.

3
Which of the following best represents the shape of a sp3 hybrid orbital of carbon? 
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4
3
4
Covalent bonding
A)involves a transfer of electrons from one atom to another.
B)occurs when atoms share all their valence electrons.
C)occurs when unpaired valence electrons are shared between atoms.
D)occurs when nonvalence electrons are shared between atoms.
E)none of these
A)involves a transfer of electrons from one atom to another.
B)occurs when atoms share all their valence electrons.
C)occurs when unpaired valence electrons are shared between atoms.
D)occurs when nonvalence electrons are shared between atoms.
E)none of these

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
5
Instructions: Propose a structure for a molecule that meets the following description.
Refer to instructions.Contains only two sp3 hybridized carbons and two sp hybridized carbons.
Refer to instructions.Contains only two sp3 hybridized carbons and two sp hybridized carbons.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
6
Instructions: Determine the hybridization for the indicated atoms in each structure below.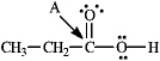
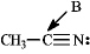
Refer to instructions.The hybridization of carbon atom A is _____.
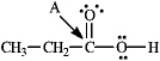
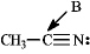
Refer to instructions.The hybridization of carbon atom A is _____.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following best represents the shape of a 2p atomic orbital of carbon? 
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
8
According to atomic theory:
A)the nucleus is positively charged.
B)the nucleus contains both charged and uncharged particles.
C)the electrons contribute very little to the total mass of the atom.
D)the electrons are located in the atomic space outside the nucleus.
E)all of these
A)the nucleus is positively charged.
B)the nucleus contains both charged and uncharged particles.
C)the electrons contribute very little to the total mass of the atom.
D)the electrons are located in the atomic space outside the nucleus.
E)all of these

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
9
How many nonbonding electron pairs are in the structure shown below? 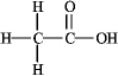
A)2
B)4
C)6
D)8
E)none of these

A)2
B)4
C)6
D)8
E)none of these

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
10
Convert the skeletal drawing of the pharmaceutical Vioxx into a molecular formula.
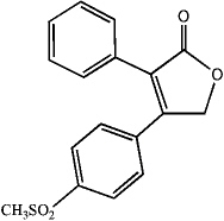


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
11
Consider the formation of an sp2 hybrid orbital.Which of the following is true?
A)Four equivalent hybrid orbitals are produced.
B)One s and one p atomic orbital are involved.
C)One p atomic orbital remains unhybridized.
D)The hybrid orbitals produced can form p bonds.
E)none of these
A)Four equivalent hybrid orbitals are produced.
B)One s and one p atomic orbital are involved.
C)One p atomic orbital remains unhybridized.
D)The hybrid orbitals produced can form p bonds.
E)none of these

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
12
Instructions: Determine the hybridization for the indicated atoms in each structure below.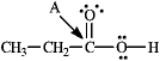
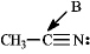
Refer to instructions.The hybridization of carbon atom B is _____.
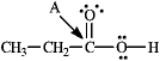
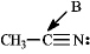
Refer to instructions.The hybridization of carbon atom B is _____.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
13
How many electrons are there in the valence shell of the carbon atom of a methyl anion,CH3-?
A)5
B)6
C)7
D)8
A)5
B)6
C)7
D)8

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
14
The structure of urea is shown below.Fill in any nonbonding valence electrons that are missing from the line-bond structure.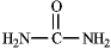
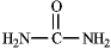

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following statements is not true?
A)The carbon-carbon single bond of an alkane is weaker than the carbon-carbon triple bond of an alkyne.
B)The carbon-carbon triple bond of an alkyne is shorter than the carbon-carbon double bond of an alkene.
C)The carbon-carbon triple bond of an alkyne is exactly three times as strong as a carbon-carbon single bond of an alkane.
D)The carbon-carbon single bond of an alkane is longer than the carbon-carbon triple bond of an alkyne.
A)The carbon-carbon single bond of an alkane is weaker than the carbon-carbon triple bond of an alkyne.
B)The carbon-carbon triple bond of an alkyne is shorter than the carbon-carbon double bond of an alkene.
C)The carbon-carbon triple bond of an alkyne is exactly three times as strong as a carbon-carbon single bond of an alkane.
D)The carbon-carbon single bond of an alkane is longer than the carbon-carbon triple bond of an alkyne.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
16
Instructions: Write valid Lewis (electron-dot)structures for each formula below.Show all electrons as dots and show all nonbonding electrons.
Write:
CH3CH2OH ethanol
Write:
CH3CH2OH ethanol

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
17
The molecular formula C2H4O can be converted into three-line bond (Kekulé)structures that are consistent with valence rules.Which one of the following Kekulé structures is not consistent with valence rules?
A)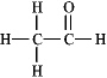
B)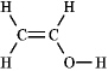
C)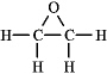
D)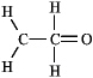
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
18
Instructions: Propose a structure for a molecule that meets the following description.
Refer to instructions.Contains only one sp3 hybridized carbon and two sp2 hybridized carbons.
Refer to instructions.Contains only one sp3 hybridized carbon and two sp2 hybridized carbons.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
19
How many total valence electrons are represented in the following electron configuration?
1s22s22px2 2py2 2pz1 or 1s22s22p5
A)1
B)3
C)5
D)7
E)9
1s22s22px2 2py2 2pz1 or 1s22s22p5
A)1
B)3
C)5
D)7
E)9

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
20
In drawing the Lewis structure for an organic compound,the carbon atoms should always be shown with
A)lone pairs of electrons.
B)four single bonds.
C)eight total electrons.
D)a positive charge.
E)none of these
A)lone pairs of electrons.
B)four single bonds.
C)eight total electrons.
D)a positive charge.
E)none of these

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
21
Draw the structure for CCl2F2 using solid,wedged,and dashed lines to show the tetrahedral geometry.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
22
The molecular orbital shown below is most likely of what type? 
A)s bonding
B)s antibonding
C)p bonding
D)p antibonding

A)s bonding
B)s antibonding
C)p bonding
D)p antibonding

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
23
Draw a picture showing the orbitals involved in the p-bonds of cyclopenta-1,3-diene,a commonly encountered reagent in organic synthesis.
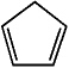


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
24
Instructions: Consider the two structures below to answer the following question.
CH3CH2OH CH3OCH3
Refer to instructions.Which of the following correctly describes the structure of these compounds?
A)All carbon atoms are sp3 hybridized.
B)All of the bonds are sigma bonds.
C)Each oxygen atom has two nonbonding pairs of electrons.
D)The bond angle around each oxygen atom is ideally about 109.5 .
E)All of these
CH3CH2OH CH3OCH3
Refer to instructions.Which of the following correctly describes the structure of these compounds?
A)All carbon atoms are sp3 hybridized.
B)All of the bonds are sigma bonds.
C)Each oxygen atom has two nonbonding pairs of electrons.
D)The bond angle around each oxygen atom is ideally about 109.5 .
E)All of these

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
25
The following species forms during an organic reaction. 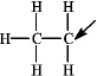
What is the formal charge on the carbon atom indicated by the arrow?
A)0
B)+1
C)-1
D)+2
E)-2

What is the formal charge on the carbon atom indicated by the arrow?
A)0
B)+1
C)-1
D)+2
E)-2

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
26
What is the expected hybridization around the sulfur atom in diethyl sulfide?
CH3CH2¾S¾CH2CH3
A)sp
B)sp2
C)sp3
D)The sulfur atom is not hybridized.
CH3CH2¾S¾CH2CH3
A)sp
B)sp2
C)sp3
D)The sulfur atom is not hybridized.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
27
Draw two possible isomers of C6H6 in which all the carbon atoms are sp2 hybridized.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following statements is not true according to molecular orbital (MO)theory?
A)Antibonding orbitals are higher in energy than the corresponding bonding orbital.
B)The head-on overlap of an s and a p atomic orbital can produce a s molecular orbital.
C)A p molecular orbital forms only from the combination of p atomic orbital wave functions.
D)The subtractive combination of atomic orbital wave functions produces a bonding molecular orbital.
A)Antibonding orbitals are higher in energy than the corresponding bonding orbital.
B)The head-on overlap of an s and a p atomic orbital can produce a s molecular orbital.
C)A p molecular orbital forms only from the combination of p atomic orbital wave functions.
D)The subtractive combination of atomic orbital wave functions produces a bonding molecular orbital.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
29
In the two structures shown below,what do the positions labeled with the arrow have in common? 
A)the same type of hybridization on the carbon atom
B)the same geometry around the carbon atom
C)the same number of hydrogen atoms bonded to the carbon atom
D)both carbon atoms are involved in a p bond

A)the same type of hybridization on the carbon atom
B)the same geometry around the carbon atom
C)the same number of hydrogen atoms bonded to the carbon atom
D)both carbon atoms are involved in a p bond

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
30
Draw all possible structures of CFnClm where n and m vary from 0 to 4.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck


