Exam 1: Structure and Bonding
Exam 1: Structure and Bonding30 Questions
Exam 2: Polar Covalent Bonds: Acids and Bases35 Questions
Exam 3: Organic Compounds: Alkanes and Their Stereochemistry32 Questions
Exam 4: Organic Compounds: Cycloalkanes and Their Stereochemistry18 Questions
Exam 5: Stereochemistry at Tetrahedral Centers33 Questions
Exam 6: An Overview of Organic Reactions32 Questions
Exam 7: Alkenes and Alkynes34 Questions
Exam 8: Reactions of Alkenes and Alkynes37 Questions
Exam 9: Aromatic Compounds36 Questions
Exam 10: Structure Determination: Mass Spectrometry,infrared Spectroscopy,and Ultraviolet Spectroscopy41 Questions
Exam 11: Structure Determination: Nuclear Magnetic Resonance Spectroscopy37 Questions
Exam 12: Organohalides: Nucleophilic Substitutions and Eliminations37 Questions
Exam 13: Alcohols,phenols,and Thiols: Ethers and Sulfides19 Questions
Exam 14: Aldehydes and Ketones: Nucleophilic Addition Reactions30 Questions
Exam 15: Carboxylic Acids and Nitriles23 Questions
Exam 16: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions42 Questions
Exam 17: Carbonyl Alpha-Substitution and Condensation Reactions84 Questions
Exam 18: Amines and Heterocycles28 Questions
Exam 19: Biomolecules: Amino Acids,peptides,and Proteins36 Questions
Exam 20: Amino Acid Metabolism34 Questions
Exam 21: Biomolecules: Carbohydrates42 Questions
Exam 22: Carbohydrate Metabolism41 Questions
Exam 23: Biomolecules: Lipids and Their Metabolism31 Questions
Exam 24: Biomolecules: Nucleic Acids and Their Metabolism23 Questions
Select questions type
The molecular orbital shown below is most likely of what type? 
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
C
Which of the following best represents the shape of a sp3 hybrid orbital of carbon? 
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
C
Instructions: Write valid Lewis (electron-dot)structures for each formula below.Show all electrons as dots and show all nonbonding electrons.
Write:
CH3CH2OH ethanol
(Essay)
4.9/5  (37)
(37)
In the two structures shown below,what do the positions labeled with the arrow have in common? 
(Multiple Choice)
4.8/5  (40)
(40)
Draw two possible isomers of C6H6 in which all the carbon atoms are sp2 hybridized.
(Essay)
4.7/5  (38)
(38)
How many nonbonding electron pairs are in the structure shown below? 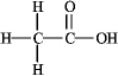
(Multiple Choice)
4.7/5  (37)
(37)
The following species forms during an organic reaction. 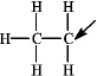 What is the formal charge on the carbon atom indicated by the arrow?
What is the formal charge on the carbon atom indicated by the arrow?
(Multiple Choice)
4.8/5  (33)
(33)
The structure of urea is shown below.Fill in any nonbonding valence electrons that are missing from the line-bond structure.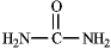
(Essay)
4.8/5  (32)
(32)
Draw a picture showing the orbitals involved in the p-bonds of cyclopenta-1,3-diene,a commonly encountered reagent in organic synthesis.
(Essay)
4.9/5  (24)
(24)
Instructions: Consider the two structures below to answer the following question.
CH3CH2OH CH3OCH3
Refer to instructions.Which of the following correctly describes the structure of these compounds?
(Multiple Choice)
4.9/5  (46)
(46)
How many total valence electrons are represented in the following electron configuration?
1s22s22px2 2py2 2pz1 or 1s22s22p5
(Multiple Choice)
4.7/5  (35)
(35)
In drawing the Lewis structure for an organic compound,the carbon atoms should always be shown with
(Multiple Choice)
4.8/5  (34)
(34)
The molecular formula C2H4O can be converted into three-line bond (Kekulé)structures that are consistent with valence rules.Which one of the following Kekulé structures is not consistent with valence rules?
(Multiple Choice)
4.9/5  (40)
(40)
What is the expected hybridization around the sulfur atom in diethyl sulfide?
CH3CH2¾S¾CH2CH3
(Multiple Choice)
4.9/5  (35)
(35)
Instructions: Propose a structure for a molecule that meets the following description.
Refer to instructions.Contains only one sp3 hybridized carbon and two sp2 hybridized carbons.
(Essay)
4.8/5  (34)
(34)
Consider the formation of an sp2 hybrid orbital.Which of the following is true?
(Multiple Choice)
4.8/5  (38)
(38)
How many electrons are there in the valence shell of the carbon atom of a methyl anion,CH3-?
(Multiple Choice)
4.8/5  (39)
(39)
Draw the structure for CCl2F2 using solid,wedged,and dashed lines to show the tetrahedral geometry.
(Essay)
4.8/5  (33)
(33)
Showing 1 - 20 of 30
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)