Deck 4: Subatomic Particles
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/146
Play
Full screen (f)
Deck 4: Subatomic Particles
1
Which of the following best describes a conceptual model of an atom?
A)a model that illustrates the tendency of an atom to undergo radioactive decay and the products it produces
B)a model that illustrates the physical structure of the atom
C)a model that represents the shape of the nucleus and the location of the electrons
D)a description of the location of the neutrons and protons in an atom
E)none of the above
A)a model that illustrates the tendency of an atom to undergo radioactive decay and the products it produces
B)a model that illustrates the physical structure of the atom
C)a model that represents the shape of the nucleus and the location of the electrons
D)a description of the location of the neutrons and protons in an atom
E)none of the above
a model that illustrates the tendency of an atom to undergo radioactive decay and the products it produces
2
Which of the following experiments helped to determine that most of an atom is actually empty space except for a very small positive core?
A)J.J. Thompson's cathode ray deflection experiments
B)R. Millikan's oil drop experiments
C)E. Rutherford's gold foil experiments
D)Avogadro's number experiments
E)Franklin's kite experiments
A)J.J. Thompson's cathode ray deflection experiments
B)R. Millikan's oil drop experiments
C)E. Rutherford's gold foil experiments
D)Avogadro's number experiments
E)Franklin's kite experiments
E. Rutherford's gold foil experiments
3
Which of the following determined the ratio of the charge of an electron to its mass?
A)J.J. Thompson's cathode ray deflection experiments
B)E. Rutherford's gold foil experiments
C)Avogadro's number experiments
D)Franklin's kite experiments
A)J.J. Thompson's cathode ray deflection experiments
B)E. Rutherford's gold foil experiments
C)Avogadro's number experiments
D)Franklin's kite experiments
J.J. Thompson's cathode ray deflection experiments
4
What prompted early scientists to propose that the ray of the cathode ray tube was due to the cathode?
A)The ray was present regardless of gas, or even without a gas.
B)The ray was attracted to positively charge electric plates.
C)The ray was not seen from the positively charged anode.
D)The ray could be diverted by a magnetic field.
E)The ray would change colors depending on which gas was used inside the tube.
A)The ray was present regardless of gas, or even without a gas.
B)The ray was attracted to positively charge electric plates.
C)The ray was not seen from the positively charged anode.
D)The ray could be diverted by a magnetic field.
E)The ray would change colors depending on which gas was used inside the tube.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
5
Would you use a physical model or a conceptual model to describe the following: a gold coin, dollar bill, car engine, air pollution, virus, spread of sexually transmitted disease?
A)conceptual model-gold coin, car engine, virus; physical model-air pollution, spread of sexually transmitted disease, dollar bill
B)physical model-gold coin, car engine, virus; conceptual model-air pollution, spread of sexually transmitted disease; dollar bill, which could represent wealth, may well be described by either model.
C)You could adequately describe all of the topics by either model. The choice depends only on the characteristics requiring description.
D)physical model-gold coin, dollar bill, car engine; conceptual model-virus, air pollution, spread of sexually transmitted disease
A)conceptual model-gold coin, car engine, virus; physical model-air pollution, spread of sexually transmitted disease, dollar bill
B)physical model-gold coin, car engine, virus; conceptual model-air pollution, spread of sexually transmitted disease; dollar bill, which could represent wealth, may well be described by either model.
C)You could adequately describe all of the topics by either model. The choice depends only on the characteristics requiring description.
D)physical model-gold coin, dollar bill, car engine; conceptual model-virus, air pollution, spread of sexually transmitted disease

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
6
The cathode ray consists of ________.
A)positively charged particles
B)negatively charged particles
C)electrically neutral particles
D)massless particles
A)positively charged particles
B)negatively charged particles
C)electrically neutral particles
D)massless particles

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
7
Why can't we see atoms?
A)We see with light energy and the wavelength of light is larger than the atom and is not reflected.
B)Atoms are invisible.
C)We cannot see things that are microscopic.
D)We see with light energy but the atoms absorb all the light and therefore there are no reflections.
E)Atoms do not interact with light energy and therefore we are unable to observe them with light.
A)We see with light energy and the wavelength of light is larger than the atom and is not reflected.
B)Atoms are invisible.
C)We cannot see things that are microscopic.
D)We see with light energy but the atoms absorb all the light and therefore there are no reflections.
E)Atoms do not interact with light energy and therefore we are unable to observe them with light.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
8
If the particles of a cathode ray had a greater electric charge, the ray passing through a magnetic field would bend ________.
A)more because of a greater electromagnetic attraction
B)less because of greater electrical inertia
C)more because the particles would be traveling faster
D)by the same amount regardless of its charge
A)more because of a greater electromagnetic attraction
B)less because of greater electrical inertia
C)more because the particles would be traveling faster
D)by the same amount regardless of its charge

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following could NOT be represented by a conceptual model?
A)the energy of a nucleus
B)the floor plan of a house
C)the weather
D)the behavior of schoolchildren
E)both A and B
A)the energy of a nucleus
B)the floor plan of a house
C)the weather
D)the behavior of schoolchildren
E)both A and B

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following experiments helped to determine the charge of an electron and (indirectly)its mass?
A)J.J. Thompson's cathode ray deflection experiments
B)R. Millikan's oil drop experiments
C)E. Rutherford's gold foil experiments
D)Avogadro's number experiments
E)Franklin's kite experiments
A)J.J. Thompson's cathode ray deflection experiments
B)R. Millikan's oil drop experiments
C)E. Rutherford's gold foil experiments
D)Avogadro's number experiments
E)Franklin's kite experiments

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
11
Would you use a physical model or a conceptual model to describe the following: the brain; the mind; the solar system; the beginning of the universe?
A)conceptual; physical; conceptual physical
B)conceptual; conceptual; conceptual; conceptual
C)physical; conceptual; physical; conceptual
D)physical; physical; physical; physical
A)conceptual; physical; conceptual physical
B)conceptual; conceptual; conceptual; conceptual
C)physical; conceptual; physical; conceptual
D)physical; physical; physical; physical

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
12
Thousands of magnetic marbles are thrown into a vertically oriented wind tunnel. As they are thrown, no marbles are free from other marbles. Instead, they clump together in groups of varying numbers. The wind tunnel operator is able to control the upward force of the wind so as to make clumps of marbles hover. She records the various forces of wind required to maintain hovering clumps in units of ounces: 45, 30, 60, 75, 105, 35, 80, 55, 90, 20, 65. From this data, what might be the weight of a single magnetic marble? What is the force of the wind analogous to within Millikan's experiment?
A)3 ounces; the force of the wind is analogous to the force of gravity within Millikan's experiment.
B)3 ounces; the force of the wind is analogous to the strength of the electric field within Millikan's experiment.
C)5 ounces; the force of the wind is analogous to the force of gravity within Millikan's experiment.
D)5 ounces; the force of the wind is analogous to the strength of the electric field within Millikan's experiment.
A)3 ounces; the force of the wind is analogous to the force of gravity within Millikan's experiment.
B)3 ounces; the force of the wind is analogous to the strength of the electric field within Millikan's experiment.
C)5 ounces; the force of the wind is analogous to the force of gravity within Millikan's experiment.
D)5 ounces; the force of the wind is analogous to the strength of the electric field within Millikan's experiment.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is not a property of an electron?
A)charge changes depending on mass
B)fundamental component of atoms
C)attracted towards positively charge electrical plates
D)weighs approximately 9.1 × 10-31 kg
E)has a negative charge
A)charge changes depending on mass
B)fundamental component of atoms
C)attracted towards positively charge electrical plates
D)weighs approximately 9.1 × 10-31 kg
E)has a negative charge

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following could best be represented by a physical model?
A)a cell
B)an atom
C)rationality
D)ecosystem
E)both A and B
A)a cell
B)an atom
C)rationality
D)ecosystem
E)both A and B

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
15
What is the main difference between a conceptual model and a physical model?
A)A physical model represents the shape and form while a conceptual model describes how a system behaves.
B)A conceptual model represents the shape and form while a physical model describes how a system behaves.
C)Physical models can only be used to represent the real world.
D)Conceptual models can only be used to describe concepts.
E)Physical models and conceptual models can be used to describe the same things.
A)A physical model represents the shape and form while a conceptual model describes how a system behaves.
B)A conceptual model represents the shape and form while a physical model describes how a system behaves.
C)Physical models can only be used to represent the real world.
D)Conceptual models can only be used to describe concepts.
E)Physical models and conceptual models can be used to describe the same things.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
16
A one ounce Ping-Pong ball is pushed upwards by a fan so that the Ping-Pong ball hovers neither rising nor falling. What is the force of the wind on the Ping-Pong ball in units of ounces?
A)1 ounce in an upward direction.
B)2 ounces in an upward direction.
C)1 ounce in an downward direction.
D)2 ounces in an downward direction.
A)1 ounce in an upward direction.
B)2 ounces in an upward direction.
C)1 ounce in an downward direction.
D)2 ounces in an downward direction.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
17
If an atom were the size of a baseball, its nucleus would be about the size of a(n)________.
A)walnut
B)raisin
C)flea
D)atom
A)walnut
B)raisin
C)flea
D)atom

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
18
What do the components of a conceptual model have in common?
A)All components interact with each other.
B)All components are mutually independent of each other.
C)The components have nothing in common. This is what differentiates a conceptual model from a physical model.
D)Each component must correlate with a corresponding component of a physical model.
A)All components interact with each other.
B)All components are mutually independent of each other.
C)The components have nothing in common. This is what differentiates a conceptual model from a physical model.
D)Each component must correlate with a corresponding component of a physical model.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
19
What prompted early scientists to propose that the ray of the cathode ray tube was actually a negatively charged particle?
A)The ray was present regardless of gas, or even without a gas.
B)The ray was attracted to positively charge electric plates.
C)The ray was not seen from the positively charged anode.
D)The ray could be diverted by a magnetic field.
E)The ray would change colors depending on which gas was used inside the tube.
A)The ray was present regardless of gas, or even without a gas.
B)The ray was attracted to positively charge electric plates.
C)The ray was not seen from the positively charged anode.
D)The ray could be diverted by a magnetic field.
E)The ray would change colors depending on which gas was used inside the tube.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
20
Since atoms are mostly empty space, why don't objects pass through one another?
A)The electrons on the atoms repel other electrons on other atoms when they get close.
B)The nucleus of one atom repels the nucleus of another atom when it gets close.
C)The nucleus of one atom attracts the nucleus of a neighboring atom to form a barrier.
D)The electrons of one attracts the nucleus of a neighboring atom to form a barrier.
E)none of the above
A)The electrons on the atoms repel other electrons on other atoms when they get close.
B)The nucleus of one atom repels the nucleus of another atom when it gets close.
C)The nucleus of one atom attracts the nucleus of a neighboring atom to form a barrier.
D)The electrons of one attracts the nucleus of a neighboring atom to form a barrier.
E)none of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
21
The ray of light within a neon sign bends when a magnet is held up to it because ________.
A)neon, like iron, is attracted to magnets
B)the light arises from the flow of electron within the tube
C)magnetic fields are able to pass through glass
D)impurities within the neon plasma are attracted to the magnet
A)neon, like iron, is attracted to magnets
B)the light arises from the flow of electron within the tube
C)magnetic fields are able to pass through glass
D)impurities within the neon plasma are attracted to the magnet

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
22
What does the following element description actually mean? 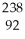 U
U
A)a uranium atom with 92 protons and 146 neutrons
B)a uranium atom with 238 neutrons and 92 protons
C)a uranium atom with 92 neutrons and 238 protons
D)a uranium atom with 92 neutrons and 146 protons
E)none of the above
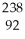 U
UA)a uranium atom with 92 protons and 146 neutrons
B)a uranium atom with 238 neutrons and 92 protons
C)a uranium atom with 92 neutrons and 238 protons
D)a uranium atom with 92 neutrons and 146 protons
E)none of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
23
Which element has the atomic number 9?
A)F
B)Ne
C)B
D)Na
E)Be
A)F
B)Ne
C)B
D)Na
E)Be

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
24
The following statement describes which subatomic particle best? It does not have an electrical charge.
A)an electron
B)a proton
C)a neutron
D)A and B
E)B and C
A)an electron
B)a proton
C)a neutron
D)A and B
E)B and C

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
25
What does the following element description actually mean? iron-57
A)iron with a mass number of 57
B)iron with an atomic number of 57
C)iron with 57 protons
D)iron with 57 neutrons
E)57 iron atoms
A)iron with a mass number of 57
B)iron with an atomic number of 57
C)iron with 57 protons
D)iron with 57 neutrons
E)57 iron atoms

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
26
If you remove two protons and two neutrons from a gold atom (Au), what new element is formed?
A)Ir2-
B)Au
C)Re
D)Au2-
E)Tl
A)Ir2-
B)Au
C)Re
D)Au2-
E)Tl

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
27
The following statement describes which subatomic particle best? It has a relatively large mass.
A)an electron
B)a proton
C)a neutron
D)A and B
E)B and C
A)an electron
B)a proton
C)a neutron
D)A and B
E)B and C

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
28
What does the following element description actually mean? hydrogen-2
A)a hydrogen with one neutron and one proton
B)a hydrogen with two neutrons
C)a hydrogen with two protons
D)a molecule of hydrogen gas
E)two hydrogen atoms
A)a hydrogen with one neutron and one proton
B)a hydrogen with two neutrons
C)a hydrogen with two protons
D)a molecule of hydrogen gas
E)two hydrogen atoms

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
29
Which element has the atomic number 12?
A)Mg
B)C
C)B
D)Na
E)Be
A)Mg
B)C
C)B
D)Na
E)Be

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following statements describes an isotope?
A)element with the same number of protons but a different number of neutrons
B)element with the same number of protons but a different number of electrons
C)element with the same number of neutrons but a different number of electrons
D)element with the same number of neutrons but a different number of protons
E)none of the above
A)element with the same number of protons but a different number of neutrons
B)element with the same number of protons but a different number of electrons
C)element with the same number of neutrons but a different number of electrons
D)element with the same number of neutrons but a different number of protons
E)none of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following statements does not describe a proton?
A)It orbits around the nucleus of an atom.
B)It has a positive charge equivalent but opposite of an electron's.
C)It is much more massive than an electron.
D)It is a nucleon.
E)It is attracted to negatively charged electrical plates.
A)It orbits around the nucleus of an atom.
B)It has a positive charge equivalent but opposite of an electron's.
C)It is much more massive than an electron.
D)It is a nucleon.
E)It is attracted to negatively charged electrical plates.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
32
What does the following element description actually mean? 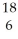 O
O
A)an oxygen atom with 6 protons and 12 neutrons
B)an oxygen atom with 6 neutrons and 12 protons
C)6 oxygen atoms with 18 neutrons
D)18 oxygen molecules with 6 neutrons each
E)none of the above
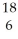 O
OA)an oxygen atom with 6 protons and 12 neutrons
B)an oxygen atom with 6 neutrons and 12 protons
C)6 oxygen atoms with 18 neutrons
D)18 oxygen molecules with 6 neutrons each
E)none of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
33
The following statement describes which subatomic particle best? It is electrically charged.
A)an electron
B)a proton
C)a neutron
D)A and B
E)B and C
A)an electron
B)a proton
C)a neutron
D)A and B
E)B and C

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
34
The following statement describes which subatomic particle best? It is a nucleon.
A)an electron
B)a proton
C)a neutron
D)A and B
E)B and C
A)an electron
B)a proton
C)a neutron
D)A and B
E)B and C

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
35
In Rutherford's gold foil experiment, alpha particles ________.
A)passed through the atomic nucleus
B)touched the atomic nucleus
C)were deflected by the atomic nucleus
D)were invisible to the atomic nucleus
A)passed through the atomic nucleus
B)touched the atomic nucleus
C)were deflected by the atomic nucleus
D)were invisible to the atomic nucleus

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following does not describe a neutron?
A)It has a positive charge equivalent but opposite of an electron's.
B)It is much more massive than an electron.
C)It is a nucleon.
D)It is often associated with protons.
E)It is more difficult to detect than a proton or an electron.
A)It has a positive charge equivalent but opposite of an electron's.
B)It is much more massive than an electron.
C)It is a nucleon.
D)It is often associated with protons.
E)It is more difficult to detect than a proton or an electron.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
37
The mass number of an element is ________.
A)the sum of the protons and the neutrons
B)the sum of the electrons and the protons
C)the sum of the electrons and the neutrons
D)the sum of the isotopes
E)the number of protons
A)the sum of the protons and the neutrons
B)the sum of the electrons and the protons
C)the sum of the electrons and the neutrons
D)the sum of the isotopes
E)the number of protons

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
38
Using the following generic atom description, choose the correct method for determining the number of neutrons. 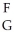 X
X
A)subtract G from F
B)subtract F from G
C)add F and G
D)divide F by G
E)look it up on the periodic table
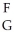 X
XA)subtract G from F
B)subtract F from G
C)add F and G
D)divide F by G
E)look it up on the periodic table

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
39
The following statement describes which subatomic particle best? It is located outside of the nucleus.
A)an electron
B)a proton
C)a neutron
D)A and B
E)B and C
A)an electron
B)a proton
C)a neutron
D)A and B
E)B and C

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
40
If you remove two protons and two electrons from a sulfur atom (S), what new element is formed?
A)Si
B)Si+2
C)Al+2
D)Al
E)Ar-2
A)Si
B)Si+2
C)Al+2
D)Al
E)Ar-2

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
41
If an element has 9 protons and 10 neutrons and 9 electrons, which expression correctly identifies the element?
A)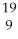 F
F
B)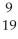 F
F
C)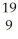 K
K
D)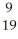 K
K
E)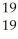 K
K
A)
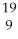 F
FB)
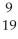 F
FC)
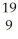 K
KD)
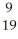 K
KE)
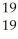 K
K
Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
42
An element has two different isotopes: one that weighs 65 amu and another that weighs 67 amu. If the average atomic mass of all the isotopes is 66.5 amu, what can be said about the relative abundance of the isotopes?
A)The isotope with the mass of 67 is more abundant than the isotope with the mass of 65.
B)The isotope with the mass of 65 is more abundant than the isotope with the mass of 67.
C)The isotope with the mass of 66.5 is more abundant than the isotope with the mass of 67.
D)The isotope with the mass of 66.5 is more abundant than the isotope with the mass of 65.
E)All the isotopes have the same relative abundance.
A)The isotope with the mass of 67 is more abundant than the isotope with the mass of 65.
B)The isotope with the mass of 65 is more abundant than the isotope with the mass of 67.
C)The isotope with the mass of 66.5 is more abundant than the isotope with the mass of 67.
D)The isotope with the mass of 66.5 is more abundant than the isotope with the mass of 65.
E)All the isotopes have the same relative abundance.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
43
How does Rutherford's model of the atom explain why some of the alpha particles directed at the gold foil were deflected straight back toward the source?
A)Alpha particles sling shot around the atomic nucleus much like comets sling shot around the sun.
B)Each atom has a center in which mass is very dense, but elastic.
C)The gold nucleus is able to deflect alpha particles much like a mirror is able to reflect photons.
D)The atomic nucleus is very small but dense and carries a positive electric charge.
A)Alpha particles sling shot around the atomic nucleus much like comets sling shot around the sun.
B)Each atom has a center in which mass is very dense, but elastic.
C)The gold nucleus is able to deflect alpha particles much like a mirror is able to reflect photons.
D)The atomic nucleus is very small but dense and carries a positive electric charge.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
44
If two protons and two neutrons are removed from the nucleus of the oxygen-16 isotope, a nucleus of which element remains?
A)nitrogen-12
B)carbon-12
C)neon-18
D)carbon-14
A)nitrogen-12
B)carbon-12
C)neon-18
D)carbon-14

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
45
You could swallow a capsule of germanium, Ge (atomic number 32), without significant ill effects. If a proton were added to each germanium nucleus, however, you would not want to swallow the capsule because the germanium would ________.
A)become arsenic
B)become radioactive
C)expand and likely lodge in your throat
D)have a change in flavor
A)become arsenic
B)become radioactive
C)expand and likely lodge in your throat
D)have a change in flavor

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
46
If an element has 10 protons and 11 neutrons and 10 electrons, which expression correctly identifies the element?
A)neon-21
B)neon-11
C)neon-31
D)sodium-11
E)sodium-20
A)neon-21
B)neon-11
C)neon-31
D)sodium-11
E)sodium-20

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
47
If a neutral element has the following chemical symbol, how many electrons does it have? 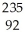 U
U
A)92
B)82
C)235
D)143
E)none of the above
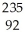 U
UA)92
B)82
C)235
D)143
E)none of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
48
If an element has 18 protons and 20 neutrons and 18 electrons, which expression correctly identifies the element?
A)argon-38
B)argon-18
C)argon-20
D)calcium-38
E)calcium-20
A)argon-38
B)argon-18
C)argon-20
D)calcium-38
E)calcium-20

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
49
Why did Rutherford propose a model for the atom with a very small nucleus containing the bulk of the mass and a positive charge?
A)because a beam of positively charged particles (alpha particles)were partially reflected by a thin gold foil
B)because a beam of positively charged particles (alpha particles)were able to penetrate by a thin gold foil
C)because a beam of negatively charged particles (beta particles)were partially reflected by a thin gold foil
D)because a beam of negatively charged particles (beta particles)were able to penetrate a thin gold foil
E)because a beam of positively charged particles (alpha particles)were completely reflected by a thin gold foil
A)because a beam of positively charged particles (alpha particles)were partially reflected by a thin gold foil
B)because a beam of positively charged particles (alpha particles)were able to penetrate by a thin gold foil
C)because a beam of negatively charged particles (beta particles)were partially reflected by a thin gold foil
D)because a beam of negatively charged particles (beta particles)were able to penetrate a thin gold foil
E)because a beam of positively charged particles (alpha particles)were completely reflected by a thin gold foil

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
50
Isotopes of the same element have a different number of ________.
A)protons
B)neutrons
C)electrons
D)photons
A)protons
B)neutrons
C)electrons
D)photons

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
51
If a neutral element has the following chemical notation, how many electrons does it have? carbon-13
A)6
B)12
C)13
D)7
E)none of the above
A)6
B)12
C)13
D)7
E)none of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
52
An element found in another galaxy exists as two isotopes. If 80.0 percent of the atoms have an atomic mass of 80.00 amu and the other 20.0 percent have an atomic mass of 82.00 amu, what is the atomic mass of the element?
A)81.0 amu
B)64.0 amu
C)80.4 amu
D)16.4 amu
E)81.6 amu
A)81.0 amu
B)64.0 amu
C)80.4 amu
D)16.4 amu
E)81.6 amu

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
53
If a neutral element has the following chemical symbol, how many electrons does it have? 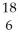 O
O
A)6
B)18
C)12
D)24
E)none of the above
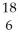 O
OA)6
B)18
C)12
D)24
E)none of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
54
Boron has primarily two isotopes, one with an atomic mass of 11.0 amu and another with an atomic mass of 10.0 amu. If the abundance of the boron atom with a mass of 11.0 amu is 18.9 percent and the abundance of the other isotope is 81.1 percent, what would be the average mass of the boron atom?
A)10.2 amu
B)11.0 amu
C)10.0 amu
D)10.8 amu
E)not enough information given
A)10.2 amu
B)11.0 amu
C)10.0 amu
D)10.8 amu
E)not enough information given

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
55
If a neutral element has the following chemical notation, how many electrons does it have? fluorine-19
A)9
B)10
C)11
D)19
E)none of the above
A)9
B)10
C)11
D)19
E)none of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
56
Does it make sense to say that a textbook is about 99.9 percent empty space?
A)No. A textbook is a solid and thus is quite dense. Therefore it is not 99.9 percent empty space.
B)No. Only gases are considered to be 99.9 percent empty space. Liquids and solids are not.
C)Yes. A textbook like all material things is made up of atoms, which are considered to be 99.9 percent empty space.
D)No. A textbook could only be considered to be 99.9 percent empty space if it were combusted.
A)No. A textbook is a solid and thus is quite dense. Therefore it is not 99.9 percent empty space.
B)No. Only gases are considered to be 99.9 percent empty space. Liquids and solids are not.
C)Yes. A textbook like all material things is made up of atoms, which are considered to be 99.9 percent empty space.
D)No. A textbook could only be considered to be 99.9 percent empty space if it were combusted.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
57
If a neutral element has 8 neutrons and 7 electrons, which expression correctly identifies the element?
A)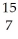 N
N
B)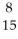 O
O
C)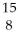 O
O
D)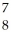 O
O
E)cannot tell from information given
A)
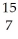 N
NB)
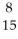 O
OC)
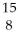 O
OD)
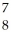 O
OE)cannot tell from information given

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
58
If an element has 15 protons and 16 neutrons and 15 electrons, what is the atomic mass of the element?
A)31
B)15
C)16
D)30
E)none of the above
A)31
B)15
C)16
D)30
E)none of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
59
Rutherford assumed that the atomic nucleus was positively charged because ________.
A)electrons were already established to be negatively charged
B)the alpha particles that ricocheted off of the nucleus are also positively charged
C)he had a very optimistic outlook on life
D)Two of the above are reasonable answers
A)electrons were already established to be negatively charged
B)the alpha particles that ricocheted off of the nucleus are also positively charged
C)he had a very optimistic outlook on life
D)Two of the above are reasonable answers

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following diagrams best represents the size of the atomic nucleus relative to the size of the atom? 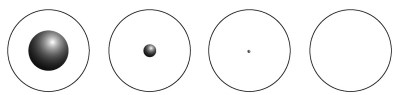 A B C D
A B C D
A)A
B)B
C)C
D)D
 A B C D
A B C DA)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following statements best describes electromagnetic radiation?
A)Light waves are oscillations of electric and magnetic fields.
B)All light energy is visible.
C)Light energy is due to vibrations in the air.
D)Light waves propagate through a medium called ether, similar to waves in water.
E)Light is due to oscillations in the nucleus of atoms.
A)Light waves are oscillations of electric and magnetic fields.
B)All light energy is visible.
C)Light energy is due to vibrations in the air.
D)Light waves propagate through a medium called ether, similar to waves in water.
E)Light is due to oscillations in the nucleus of atoms.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
62
What is the approximate mass of a carbon atom in atomic mass units (amu)? How about a carbon dioxide molecule?
A)A carbon atom has a mass of about 12 amu. Carbon dioxide would then have a mass of 28 amu.
B)A carbon atom has a mass of about 6 amu. Carbon dioxide would then have a mass of 32 amu.
C)A carbon atom has a mass of about 12 amu. Carbon dioxide would then have a mass of 44 amu.
D)A carbon atom has a mass of about 6 amu. Carbon dioxide would then have a mass of 22 amu.
A)A carbon atom has a mass of about 12 amu. Carbon dioxide would then have a mass of 28 amu.
B)A carbon atom has a mass of about 6 amu. Carbon dioxide would then have a mass of 32 amu.
C)A carbon atom has a mass of about 12 amu. Carbon dioxide would then have a mass of 44 amu.
D)A carbon atom has a mass of about 6 amu. Carbon dioxide would then have a mass of 22 amu.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
63
The nucleus of an electrically neutral iron atom contains 26 protons. How many electrons does this iron atom have?
A)52
B)26
C)24
D)none
A)52
B)26
C)24
D)none

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
64
When we breathe we inhale oxygen, 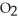 , and exhale carbon dioxide,
, and exhale carbon dioxide, 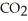 , plus water vapor,
, plus water vapor,  O. Which likely has more mass, the air that we inhale or the same volume of air we exhale? Does breathing cause you to lose or gain weight?
O. Which likely has more mass, the air that we inhale or the same volume of air we exhale? Does breathing cause you to lose or gain weight?
A)The air we inhale has more mass; Breathing causes you to lose weight.
B)The air we exhale has more mass; Breathing causes you to lose weight.
C)The air we inhale has more mass; Breathing causes you to gain weight.
D)The air we exhale has more mass; Breathing causes you to gain weight.
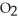 , and exhale carbon dioxide,
, and exhale carbon dioxide, 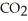 , plus water vapor,
, plus water vapor, 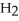 O. Which likely has more mass, the air that we inhale or the same volume of air we exhale? Does breathing cause you to lose or gain weight?
O. Which likely has more mass, the air that we inhale or the same volume of air we exhale? Does breathing cause you to lose or gain weight?A)The air we inhale has more mass; Breathing causes you to lose weight.
B)The air we exhale has more mass; Breathing causes you to lose weight.
C)The air we inhale has more mass; Breathing causes you to gain weight.
D)The air we exhale has more mass; Breathing causes you to gain weight.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
65
A beam of protons and a beam of neutrons of the same energy are both harmful to living tissue. The beam of neutrons, however, is less harmful. Why?
A)Neutrons are much smaller and lighter than protons and would do less damage.
B)Neutrons travel at reduced speed compared to the speed at which protons travel.
C)Neutrons carry no electric charge and thus have a greater likelihood of passing through the tissue.
D)All of the above are reasons why neutrons are less harmful.
A)Neutrons are much smaller and lighter than protons and would do less damage.
B)Neutrons travel at reduced speed compared to the speed at which protons travel.
C)Neutrons carry no electric charge and thus have a greater likelihood of passing through the tissue.
D)All of the above are reasons why neutrons are less harmful.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
66
In the following diagram, which of the following is the measurement of one wavelength? 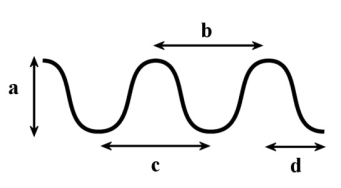
A)a
B)b
C)c
D)d
E)b and c

A)a
B)b
C)c
D)d
E)b and c

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
67
Evidence for the existence of neutrons did not come until many years after the discoveries of the electron and the proton. Give a possible explanation.
A)The neutron is nearly massless.
B)The neutron is only slightly more massive than the proton.
C)The discovery required the use of ultrafast computers.
D)The neutron lacks an electrical charge.
A)The neutron is nearly massless.
B)The neutron is only slightly more massive than the proton.
C)The discovery required the use of ultrafast computers.
D)The neutron lacks an electrical charge.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
68
The isotope lithium-7 has a mass of 7.0160 atomic mass units, and the isotope lithium-6 has a mass of 6.0151 atomic mass units. Given the information that 92.58 percent of all lithium atoms found in nature are lithium-7 and 7.42 percent are lithium-6, calculate the atomic mass of lithium, Li (atomic number 3).
A)7.0160 amu
B)6.942 amu
C)6.495 amu
D)13.031 amu
A)7.0160 amu
B)6.942 amu
C)6.495 amu
D)13.031 amu

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
69
Which contributes more to an atom's mass: electrons or protons? Which contributes more to an atom's size?
A)Protons contribute more to an atom's mass while electrons contribute more to its size.
B)Electrons contribute more to an atom's mass while protons contribute more to its size.
C)Protons contribute more to both the mass and size of an atom.
D)Electrons contribute more to both the mass and size of an atom.
A)Protons contribute more to an atom's mass while electrons contribute more to its size.
B)Electrons contribute more to an atom's mass while protons contribute more to its size.
C)Protons contribute more to both the mass and size of an atom.
D)Electrons contribute more to both the mass and size of an atom.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
70
Which of these shows light waves in order of increasing energy?
A)Radio < Microwave < Infrared < Ultraviolet < Visible
B)Visible < Ultraviolet < Microwave < Infrared < Radio
C)Ultraviolet < Visible < Infrared < Microwave < Radio
D)Radio < Microwave < Infrared < Visible < Ultraviolet
A)Radio < Microwave < Infrared < Ultraviolet < Visible
B)Visible < Ultraviolet < Microwave < Infrared < Radio
C)Ultraviolet < Visible < Infrared < Microwave < Radio
D)Radio < Microwave < Infrared < Visible < Ultraviolet

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
71
Why are the atomic masses listed in the periodic table not whole numbers?
A)Scientists have yet to make the precise measurements.
B)That would be too much of a coincidence.
C)The atomic masses are average atomic masses.
D)Today's instruments are able to measure the atomic masses to many decimal places.
A)Scientists have yet to make the precise measurements.
B)That would be too much of a coincidence.
C)The atomic masses are average atomic masses.
D)Today's instruments are able to measure the atomic masses to many decimal places.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
72
How are frequency and wavelength related?
A)The higher the frequency the lower the wavelength.
B)The larger the wavelength the higher the frequency.
C)The lower the frequency the lower the wavelength.
D)wavelength = frequency
E)none of the above
A)The higher the frequency the lower the wavelength.
B)The larger the wavelength the higher the frequency.
C)The lower the frequency the lower the wavelength.
D)wavelength = frequency
E)none of the above

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
73
If an atom has 43 electrons, 56 neutrons, and 43 protons, what is its approximate atomic mass? What is the name of this element?
A)atomic mass, 137 amu; Barium
B)atomic mass, 99 amu; Technetium
C)atomic mass, 99 amu; Radon
D)atomic mass 142 amu; Einsteinium
A)atomic mass, 137 amu; Barium
B)atomic mass, 99 amu; Technetium
C)atomic mass, 99 amu; Radon
D)atomic mass 142 amu; Einsteinium

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
74
What is the difference between radiation and electromagnetic radiation?
A)Radiation is from decay of nuclei while electromagnetic radiation is any form of light.
B)Radiation is particles while electromagnetic radiation is a wave.
C)Radiation can be blocked by lead plates while electromagnetic radiation cannot be.
D)Radiation is waves while electromagnetic radiation is a particle.
E)Radiation is damaging to living tissue while electromagnetic radiation has no effect on living tissue.
A)Radiation is from decay of nuclei while electromagnetic radiation is any form of light.
B)Radiation is particles while electromagnetic radiation is a wave.
C)Radiation can be blocked by lead plates while electromagnetic radiation cannot be.
D)Radiation is waves while electromagnetic radiation is a particle.
E)Radiation is damaging to living tissue while electromagnetic radiation has no effect on living tissue.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
75
Which has more atoms: a 1-gram sample of carbon-12 or a 1-gram sample of carbon-13?
A)More information is needed.
B)a 1-gram sample of carbon-12
C)a 1-gram sample of carbon-13
D)They have the same number of atoms.
A)More information is needed.
B)a 1-gram sample of carbon-12
C)a 1-gram sample of carbon-13
D)They have the same number of atoms.

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
76
In the following diagram, which of the following measurements would represent the distance the wave travels in 1 second if it has a frequency of 0.5 Hz? 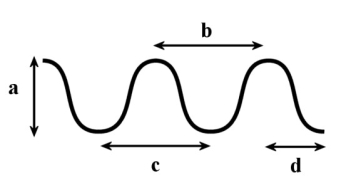
A)a
B)b
C)c
D)d
E)b and c

A)a
B)b
C)c
D)d
E)b and c

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the above has the most energy?
A)a
B)b
C)c
D)d
E)e
A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the above has the highest wavelength?
A)a
B)b
C)c
D)d
E)e
A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the above has the highest frequency?
A)a
B)b
C)c
D)d
E)e
A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck
80
The element bromine, Br (atomic number 35), has two major isotopes of similar abundance, both around 50 percent. The atomic mass of bromine is reported in the periodic table as 79.904 atomic mass units. Choose the most likely set of mass numbers for these two bromine isotopes.
A)Br-80, Br-81
B)Br-79, Br-80
C)Br-79, Br-81
D)Br-78, Br-80
A)Br-80, Br-81
B)Br-79, Br-80
C)Br-79, Br-81
D)Br-78, Br-80

Unlock Deck
Unlock for access to all 146 flashcards in this deck.
Unlock Deck
k this deck


