Exam 4: Subatomic Particles
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds162 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
The electron configuration of any neutral group 1 element always ends with ________.
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
A
What vibrates within an atom as it emits electromagnetic waves?
Free
(Multiple Choice)
4.7/5  (43)
(43)
Correct Answer:
A
Thousands of magnetic marbles are thrown into a vertically oriented wind tunnel. As they are thrown, no marbles are free from other marbles. Instead, they clump together in groups of varying numbers. The wind tunnel operator is able to control the upward force of the wind so as to make clumps of marbles hover. She records the various forces of wind required to maintain hovering clumps in units of ounces: 45, 30, 60, 75, 105, 35, 80, 55, 90, 20, 65. From this data, what might be the weight of a single magnetic marble? What is the force of the wind analogous to within Millikan's experiment?
Free
(Multiple Choice)
5.0/5  (35)
(35)
Correct Answer:
D
Light is emitted as an electron transitions from a higher energy state to a lower energy state. How long does it take for the actual transition to take place?
(Multiple Choice)
4.8/5  (36)
(36)
Which loses its outermost electrons more easily: bromine, Br, or krypton, Kr?
(Multiple Choice)
4.8/5  (32)
(32)
You could swallow a capsule of germanium, Ge (atomic number 32), without significant ill effects. If a proton were added to each germanium nucleus, however, you would not want to swallow the capsule because the germanium would ________.
(Multiple Choice)
4.8/5  (39)
(39)
An electron de-excites from the fourth quantum level to the third and then directly to the first. Two frequencies of light are emitted. How do their combined energies compare to the energy of the single frequency that would be emitted by de-excitation from the fourth level directly to the first level? The combined energies of the two frequencies emitted by the one electrons is ________.
(Multiple Choice)
4.8/5  (37)
(37)
Visible light has a frequency range between 7 × 1014 Hz and 4 × 1014 Hz. Which frequency of light would have the most energy?
(Multiple Choice)
4.9/5  (39)
(39)
Which experiences a greater effective nuclear charge, an electron in the outer shell of neon (atomic number 10)or an electron in the outer shell of sodium (atomic number 11)?
(Multiple Choice)
4.8/5  (30)
(30)
The ________ represents the complete range of frequencies of light energy from radio waves to gamma rays.
(Multiple Choice)
4.9/5  (39)
(39)
List the following atoms in order of increasing atomic size: Thallium, Tl; Germanium, Ge; Tin, Sn; Phosphorus, P.
(Multiple Choice)
4.8/5  (47)
(47)
What do the components of a conceptual model have in common?
(Multiple Choice)
4.8/5  (34)
(34)
How might you distinguish a sodium-vapor street lamp from a mercury-vapor street lamp?
(Multiple Choice)
4.7/5  (40)
(40)
Rank the following by relative frequency from highest to lowest:
(Multiple Choice)
4.9/5  (35)
(35)
Consider the various frequencies of the three photons emitted from the following three individual electron transitions in the figure below: n=3 to n=2; n=2 to n=1; n=3 to n=1. These transitions would produce three spectral lines in a spectroscope. If the energy spacing between the levels were equal, would this affect the number of spectral lines? 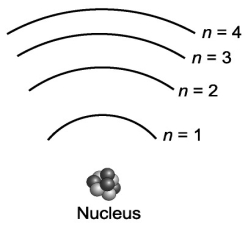
(Multiple Choice)
4.8/5  (42)
(42)
From which of the following atoms would removing an electron be the most difficult?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following concepts underlies all the others: ionization energy, effective nuclear charge, atomic size.
(Multiple Choice)
4.8/5  (37)
(37)
Which contributes more to an atom's mass: electrons or protons? Which contributes more to an atom's size?
(Multiple Choice)
4.8/5  (38)
(38)
Isotopes of the same element have a different number of ________.
(Multiple Choice)
4.7/5  (40)
(40)
Showing 1 - 20 of 146
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)