Deck 10: Acids and Bases in Our Environment
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/135
Play
Full screen (f)
Deck 10: Acids and Bases in Our Environment
1
What is a base?
A)anything that accepts a hydrogen ion
B)anything that accepts a hydroxide ion
C)anything that donates a hydroxide ion
D)anything that can be used to clean drains
E)anything with a bitter taste
A)anything that accepts a hydrogen ion
B)anything that accepts a hydroxide ion
C)anything that donates a hydroxide ion
D)anything that can be used to clean drains
E)anything with a bitter taste
anything that accepts a hydrogen ion
2
For the following reaction, identify whether the compound in bold is behaving as an acid or a base. H3PO4 + H2O ⇌ H2PO4- + H3O+
A)acid
B)base
C)neither
D)both
E)none of the above
A)acid
B)base
C)neither
D)both
E)none of the above
base
3
According to the following reaction, which molecule is acting as a base? OH- + NH4+ → H2O + NH3
A)H2O
B)NH3
C)OH-
D)NH4+
E)none of the above
A)H2O
B)NH3
C)OH-
D)NH4+
E)none of the above
OH-
4
The hydronium ion, H3O+, is a ________.
A)unique form of water
B)very strong base
C)polyatomic ion
D)proton acceptor
A)unique form of water
B)very strong base
C)polyatomic ion
D)proton acceptor

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
5
For the following reaction, identify whether the compound in bold is behaving as an acid or a base. H3PO4 + H2O ⇌ H2PO4- + H3O+
A)acid
B)base
C)neither
D)both
E)none of the above
A)acid
B)base
C)neither
D)both
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
6
How do you make a proton out of a hydrogen atom?
A)remove an electron from a hydrogen atom
B)remove a proton from a helium nucleus
C)let the hydrogen atoms undergo fusion
D)let the hydrogen atoms combine to form a hydrogen molecule and eject an electron
E)let the hydrogen atoms combine to form a hydrogen molecule and eject a proton
A)remove an electron from a hydrogen atom
B)remove a proton from a helium nucleus
C)let the hydrogen atoms undergo fusion
D)let the hydrogen atoms combine to form a hydrogen molecule and eject an electron
E)let the hydrogen atoms combine to form a hydrogen molecule and eject a proton

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
7
According to the following reaction, which molecule is acting as an acid? H2O + NH3 → OH- + NH4+
A)H2O
B)NH3
C)OH-
D)NH4+
E)none of the above
A)H2O
B)NH3
C)OH-
D)NH4+
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
8
What is an acid?
A)anything that donates hydrogen ions
B)anything that accepts hydrogen atoms
C)anything that donates hydrogen atoms
D)anything that dissolves metal
E)anything that donates hydronium ions
A)anything that donates hydrogen ions
B)anything that accepts hydrogen atoms
C)anything that donates hydrogen atoms
D)anything that dissolves metal
E)anything that donates hydronium ions

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
9
For the following reaction, identify whether the compound in bold is behaving as an acid or a base. H3PO4 + H2O ⇌ H2PO4- + H3O+
A)acid
B)base
C)neither
D)both
E)none of the above
A)acid
B)base
C)neither
D)both
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
10
According to the following reaction, which molecule is acting as an acid? OH- + NH4+ → H2O + NH3
A)H2O
B)NH3
C)OH-
D)NH4+
E)none of the above
A)H2O
B)NH3
C)OH-
D)NH4+
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
11
What best describes what happens when an acid such as HCl is mixed with water?
A)The proton chemically bonded to the chlorine is transferred to a water molecule and forms a chloride ion and a hydronium ion.
B)A proton from the chlorine nucleus is ejected and captured by a water molecule to form a negatively charged HCl and a new hydronium ion.
C)A hydroxide ion from the water is transferred to the HCl molecule to form a proton and hydronium ion.
D)HCl is not an acid.
E)none of the above
A)The proton chemically bonded to the chlorine is transferred to a water molecule and forms a chloride ion and a hydronium ion.
B)A proton from the chlorine nucleus is ejected and captured by a water molecule to form a negatively charged HCl and a new hydronium ion.
C)A hydroxide ion from the water is transferred to the HCl molecule to form a proton and hydronium ion.
D)HCl is not an acid.
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
12
According to the following reaction, which molecule is acting as a base? H2O + H2SO4 → H3O+ + HSO4-
A)H2SO4
B)H2O
C)H3O+
D)HSO4-
E)none of the above
A)H2SO4
B)H2O
C)H3O+
D)HSO4-
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
13
According to the following reaction, which molecule is acting as a base? H3O+ + HSO4- → H2O + H2SO4
A)H2SO4
B)H2O
C)H3O+
D)HSO4-
E)none of the above
A)H2SO4
B)H2O
C)H3O+
D)HSO4-
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
14
For the following reaction, identify whether the compound in bold is behaving as an acid or a base. H3PO4 + H2O ⇌ H2PO4- + H3O+
A)acid
B)base
C)neither
D)both
E)none of the above
A)acid
B)base
C)neither
D)both
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
15
According to the following reaction, which molecule is acting as a base? H2O + NH3 → OH- + NH4+
A)H2O
B)NH3
C)OH-
D)NH4+
E)none of the above
A)H2O
B)NH3
C)OH-
D)NH4+
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
16
In the reaction below, what does the symbol ⇌ mean? OH- + NH4+ ⇌ H2O + NH3
A)It means that the forward and backward reactions are happening at the same time.
B)It means that the reaction cannot decide which way to go.
C)It means that the forward reaction does not progress.
D)It means that the backward reaction happens as fast as the forward reaction and so the proton is not transferred at all.
E)none of the above
A)It means that the forward and backward reactions are happening at the same time.
B)It means that the reaction cannot decide which way to go.
C)It means that the forward reaction does not progress.
D)It means that the backward reaction happens as fast as the forward reaction and so the proton is not transferred at all.
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
17
The hydroxide ion, HO-, is a ________.
A)unique form of water
B)very strong acid
C)polyatomic ion
D)proton donator
A)unique form of water
B)very strong acid
C)polyatomic ion
D)proton donator

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following compounds could never act as an acid?
A)SO4-2
B)HSO4-1
C)H2SO4
D)NH3
E)CH3CO2H
A)SO4-2
B)HSO4-1
C)H2SO4
D)NH3
E)CH3CO2H

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
19
According to the following reaction, which molecule is acting as an acid? H3O+ + HSO4- → H2O + H2SO4
A)H2SO4
B)H2O
C)H3O+
D)HSO4-
E)none of the above
A)H2SO4
B)H2O
C)H3O+
D)HSO4-
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
20
According to the following reaction, which molecule is acting as an acid? H2O + H2SO4 → H3O+ + HSO4-
A)H2SO4
B)H2O
C)H3O+
D)HSO4-
E)none of the above
A)H2SO4
B)H2O
C)H3O+
D)HSO4-
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
21
Does the hydride ion, H⁻, tend to behave as an acid or a base?
A)Hydride ion is unstable and re-reacts with water to form an acid in the form of the hydronium ion, H3O⁺.
B)Hydride ion has a pair of electrons and acts like a Lewis base since it has a strong tendency to lend or donate that electron pair.
C)Although the hydride ion has two electrons, they do not pair together. Thus, the hydride ion is incapable of acting as a Lewis base and therefore tends to behave as an acid.
D)Hydride ion's makeup consists of one proton and two electrons. As such, its tendency is to form neutral hydrogen gas,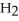 , and therefore is neither acidic nor basic, but neutral.
, and therefore is neither acidic nor basic, but neutral.
A)Hydride ion is unstable and re-reacts with water to form an acid in the form of the hydronium ion, H3O⁺.
B)Hydride ion has a pair of electrons and acts like a Lewis base since it has a strong tendency to lend or donate that electron pair.
C)Although the hydride ion has two electrons, they do not pair together. Thus, the hydride ion is incapable of acting as a Lewis base and therefore tends to behave as an acid.
D)Hydride ion's makeup consists of one proton and two electrons. As such, its tendency is to form neutral hydrogen gas,
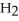 , and therefore is neither acidic nor basic, but neutral.
, and therefore is neither acidic nor basic, but neutral.
Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
22
For the following acid-base reaction, identify what is formed in the space marked. HF + KOH ⇌ ???? + H2O
A)KF
B)H3OF
C)KOH2
D)KOH2F
E)none of the above
A)KF
B)H3OF
C)KOH2
D)KOH2F
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
23
For the following acid-base reaction, identify what is formed in the space marked. H2SO4 + KOH ⇌ KHSO4 + ???
A)K2SO4
B)H3OSO4
C)H2O
D)KOH2SO4
E)none of the above
A)K2SO4
B)H3OSO4
C)H2O
D)KOH2SO4
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
24
The main component of bleach is sodium hypochlorite, NaOCl, which consists of sodium ions, Na⁺, and hypochlorite ions, 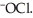 What products are formed when this compound is reacted with the hydrochloric acid, HCl, of toilet bowl cleaner?
What products are formed when this compound is reacted with the hydrochloric acid, HCl, of toilet bowl cleaner?
A)The products are NaCl,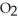 , and
, and 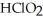 .
.
B)The products are NaOH,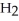 O, and
O, and 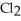
C)The products are NaCl and HOCl.
D)The products are NaOH,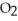 , and
, and 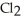
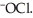 What products are formed when this compound is reacted with the hydrochloric acid, HCl, of toilet bowl cleaner?
What products are formed when this compound is reacted with the hydrochloric acid, HCl, of toilet bowl cleaner?A)The products are NaCl,
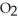 , and
, and 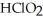 .
.B)The products are NaOH,
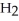 O, and
O, and 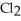
C)The products are NaCl and HOCl.
D)The products are NaOH,
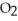 , and
, and 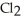

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
25
Suggest why people once washed their hands with ashes.
A)The ashes act as a base and reacts with skin oils to produce solutions of soap.
B)The ashes act as an acid and reacts with the skin oils to produce soap.
C)The oils on the skin act as a base and reacts with the ashes to produce soap.
D)After being burnt in the fire, the acids and bases of the log are neutralized, making it a gentle material to use on the hands.
A)The ashes act as a base and reacts with skin oils to produce solutions of soap.
B)The ashes act as an acid and reacts with the skin oils to produce soap.
C)The oils on the skin act as a base and reacts with the ashes to produce soap.
D)After being burnt in the fire, the acids and bases of the log are neutralized, making it a gentle material to use on the hands.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
26
What atom in the ammonium ion, 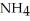 ⁺ bears the positive charge?
⁺ bears the positive charge?
A)the nitrogen atom
B)the hydrogen atom
C)Neither atom bears a positive charge.
D)Nitrogen and hydrogen equally share the positive charge.
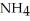 ⁺ bears the positive charge?
⁺ bears the positive charge?A)the nitrogen atom
B)the hydrogen atom
C)Neither atom bears a positive charge.
D)Nitrogen and hydrogen equally share the positive charge.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
27
Identify the acid or base behavior of each substance in these reactions: 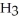
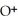 +
+ 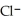 ⇌
⇌ 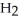 O + HCl
O + HCl
A)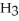
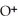 acts as an acid,
acts as an acid, 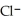 acts as a base,
acts as a base, 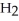 O acts an acid, HCl acts as a base.
O acts an acid, HCl acts as a base.
B)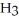
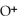 acts as an base,
acts as an base, 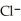 acts as a acid,
acts as a acid, 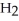 O acts an acid, HCl acts as a base.
O acts an acid, HCl acts as a base.
C)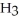
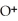 acts as an acid,
acts as an acid, 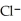 acts as a base,
acts as a base, 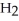 O acts an base, HCl acts as a acid.
O acts an base, HCl acts as a acid.
D)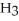
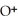 acts as an base,
acts as an base, 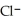 acts as a acid,
acts as a acid, 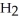 O acts an base, HCl acts as an acid.
O acts an base, HCl acts as an acid.
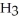
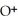 +
+ 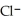 ⇌
⇌ 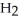 O + HCl
O + HClA)
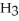
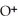 acts as an acid,
acts as an acid, 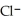 acts as a base,
acts as a base, 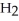 O acts an acid, HCl acts as a base.
O acts an acid, HCl acts as a base.B)
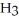
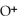 acts as an base,
acts as an base, 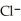 acts as a acid,
acts as a acid, 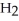 O acts an acid, HCl acts as a base.
O acts an acid, HCl acts as a base.C)
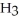
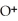 acts as an acid,
acts as an acid, 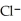 acts as a base,
acts as a base, 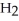 O acts an base, HCl acts as a acid.
O acts an base, HCl acts as a acid.D)
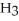
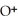 acts as an base,
acts as an base, 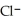 acts as a acid,
acts as a acid, 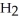 O acts an base, HCl acts as an acid.
O acts an base, HCl acts as an acid.
Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
28
What happens to the corrosive properties of an acid and a base after they neutralize each other? Why?
A)The corrosive properties are neutralized because the acid and base no longer exist.
B)The corrosive properties are unaffected because salt is a corrosive agent.
C)The corrosive properties are doubled because the acid and base are combined in the salt.
D)The corrosive properties remain the same when the salt is mixed into water.
A)The corrosive properties are neutralized because the acid and base no longer exist.
B)The corrosive properties are unaffected because salt is a corrosive agent.
C)The corrosive properties are doubled because the acid and base are combined in the salt.
D)The corrosive properties remain the same when the salt is mixed into water.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
29
Identify the acid or base behavior of each substance in these reactions: HS 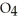
 +
+ 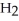 O ⇌ O
O ⇌ O 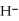 +
+ 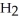 S
S 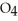
A)HS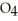
 acts as an acid,
acts as an acid, 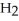 O acts as a base, O
O acts as a base, O 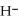 acts as an acid,
acts as an acid, 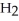 S
S 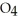 acts as a base.
acts as a base.
B)HS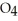
 acts as an base,
acts as an base, 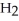 O acts as a acid, O
O acts as a acid, O 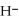 acts as an acid,
acts as an acid, 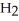 S
S 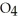 acts as a base.
acts as a base.
C)HS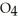
 acts as an acid,
acts as an acid, 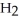 O acts as a base, O
O acts as a base, O 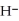 acts as an base,
acts as an base, 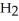 S
S 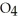 acts as a acid.
acts as a acid.
D)HS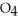
 acts as an base,
acts as an base, 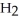 O acts as a acid, O
O acts as a acid, O 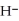 acts as an base,
acts as an base, 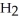 S
S 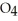 acts as a acid.
acts as a acid.
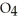
 +
+ 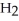 O ⇌ O
O ⇌ O 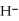 +
+ 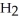 S
S 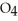
A)HS
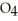
 acts as an acid,
acts as an acid, 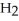 O acts as a base, O
O acts as a base, O 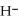 acts as an acid,
acts as an acid, 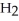 S
S 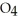 acts as a base.
acts as a base.B)HS
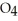
 acts as an base,
acts as an base, 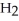 O acts as a acid, O
O acts as a acid, O 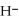 acts as an acid,
acts as an acid, 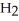 S
S 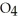 acts as a base.
acts as a base.C)HS
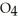
 acts as an acid,
acts as an acid, 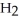 O acts as a base, O
O acts as a base, O 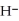 acts as an base,
acts as an base, 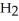 S
S 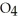 acts as a acid.
acts as a acid.D)HS
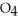
 acts as an base,
acts as an base, 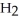 O acts as a acid, O
O acts as a acid, O 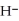 acts as an base,
acts as an base, 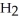 S
S 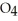 acts as a acid.
acts as a acid.
Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
30
A strong acid tends ________.
A)to be strongly polar
B)to form negatively charged ions when dissolved in water
C)to form positively charged ions when dissolved in water
D)all of the above
A)to be strongly polar
B)to form negatively charged ions when dissolved in water
C)to form positively charged ions when dissolved in water
D)all of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
31
Water is formed from the reaction of an acid and a base. Why is it not classified as a salt?
A)Not all acid base reactions produce a salt, as in the case with the formation of water.
B)The attraction between the two ions in water molecules are too strong.
C)By definition, a salt must be able to dissolve in water, so water itself cannot be called a salt.
D)A salt is an ionic compound, whereas water is a covalent compound.
A)Not all acid base reactions produce a salt, as in the case with the formation of water.
B)The attraction between the two ions in water molecules are too strong.
C)By definition, a salt must be able to dissolve in water, so water itself cannot be called a salt.
D)A salt is an ionic compound, whereas water is a covalent compound.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following statements about strong or weak acids is true?
A)A weak acid and a strong acid at the same concentration are equally corrosive.
B)A weak acid readily forms ions when dissolved in water.
C)A strong acid will never react with a strong base.
D)A weak acid will react with a strong base.
A)A weak acid and a strong acid at the same concentration are equally corrosive.
B)A weak acid readily forms ions when dissolved in water.
C)A strong acid will never react with a strong base.
D)A weak acid will react with a strong base.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following statements is not true about a neutralization reaction?
A)Water is always formed in a neutralization reaction.
B)One molecule of acid neutralizes one molecule of base.
C)A neutralization is the reaction of a hydroxide ion with a proton.
D)All of the above are true.
E)None of the above are true.
A)Water is always formed in a neutralization reaction.
B)One molecule of acid neutralizes one molecule of base.
C)A neutralization is the reaction of a hydroxide ion with a proton.
D)All of the above are true.
E)None of the above are true.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
34
For the following acid-base reaction, identify what compound is formed in the space marked. HCl + KOH ⇌ ???? + H2O
A)KCl
B)H3OCl
C)KOH2
D)KOH2Cl
E)none of the above
A)KCl
B)H3OCl
C)KOH2
D)KOH2Cl
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
35
For the following acid-base reaction, identify what compound is formed in the space marked. HNO3 + KOH ⇌ ???? + H2O
A)KNO3
B)H3ONO3
C)KOH2
D)KOH2NO3
E)none of the above
A)KNO3
B)H3ONO3
C)KOH2
D)KOH2NO3
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
36
For the following acid-base reaction, identify which salt is formed. HCl + NaOH ⇌ ???? + H2O
A)NaCl
B)H3OCl
C)NaOH2
D)NaOH2Cl
E)none of the above
A)NaCl
B)H3OCl
C)NaOH2
D)NaOH2Cl
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
37
What atom in the hydronium ion, H3O⁺, bears the positive charge?
A)the hydrogen atom
B)the oxygen atom
C)Neither atom bears a positive charge.
D)Hydrogen and oxygen equally share the positive charge.
A)the hydrogen atom
B)the oxygen atom
C)Neither atom bears a positive charge.
D)Hydrogen and oxygen equally share the positive charge.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
38
What is the relationship between the hydroxide ion and a water molecule?
A)A hydroxide ion is a water molecule plus a proton.
B)A hydroxide ion and a water molecule are the same things.
C)A hydroxide ion is a water molecule minus a hydrogen nucleus.
D)A hydroxide ion is a water molecule plus two extra electrons.
A)A hydroxide ion is a water molecule plus a proton.
B)A hydroxide ion and a water molecule are the same things.
C)A hydroxide ion is a water molecule minus a hydrogen nucleus.
D)A hydroxide ion is a water molecule plus two extra electrons.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
39
An acid and a base react to form a salt, which consists of positive and negative ions. Which forms the positive ions: the acid or the base? Which forms the negative ions?
A)The acid forms the positive ion, the base forms the negative ion.
B)The acid forms the negative ion, the base forms the positive ion.
C)Because different substances can act as an acid or a base, it depends on the substance you begin with.
D)all of the above
A)The acid forms the positive ion, the base forms the negative ion.
B)The acid forms the negative ion, the base forms the positive ion.
C)Because different substances can act as an acid or a base, it depends on the substance you begin with.
D)all of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
40
What results when water, 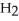 O, reacts with sulfur dioxide, S
O, reacts with sulfur dioxide, S 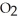 ?
?
A)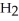 O + S
O + S 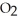 →
→ 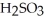 (aq)
(aq)
B)2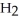 O + 2 S
O + 2 S 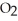 → 2
→ 2 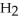
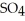 (aq)+
(aq)+ 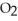 (g)
(g)
C)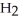 O + S
O + S 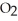 → S
→ S 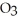 (g)+
(g)+ 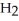 (g)
(g)
D)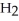 O + S
O + S 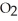 → No Reaction
→ No Reaction
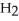 O, reacts with sulfur dioxide, S
O, reacts with sulfur dioxide, S 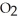 ?
?A)
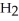 O + S
O + S 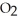 →
→ 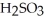 (aq)
(aq)B)2
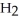 O + 2 S
O + 2 S 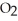 → 2
→ 2 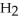
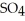 (aq)+
(aq)+ 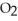 (g)
(g)C)
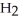 O + S
O + S 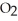 → S
→ S 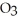 (g)+
(g)+ 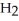 (g)
(g)D)
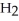 O + S
O + S 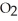 → No Reaction
→ No Reaction
Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
41
How readily an acid donates a hydrogen ion is a function of how well the acid is able to accommodate the resulting negative charge it gains after donating. Which should be the stronger acid: water or hypochlorous acid? Why? 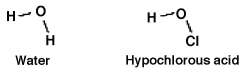
A)Water, because O-H would accommodate the resulting negative charge better than O-Cl.
B)Hypochlorous acid, because the resulting negative charge is spread over a greater number of atoms.
C)Neither, since both molecules are "monoprotic" and donate the same H+ ion, making their the same.
D)Water, because the hydrogen bonding in water gives it the edge in the ability to accommodate the resulting negative charge.

A)Water, because O-H would accommodate the resulting negative charge better than O-Cl.
B)Hypochlorous acid, because the resulting negative charge is spread over a greater number of atoms.
C)Neither, since both molecules are "monoprotic" and donate the same H+ ion, making their the same.
D)Water, because the hydrogen bonding in water gives it the edge in the ability to accommodate the resulting negative charge.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
42
Why are aqueous solutions of highly charged metal ions, such as 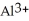 , usually acidic?
, usually acidic?
A)The highly charged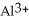 ion strips hydrogen ions away from water as if forms
ion strips hydrogen ions away from water as if forms 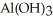 and releases them into the solution causing the solution to become acidic.
and releases them into the solution causing the solution to become acidic.
B)As the water molecule binds to the aluminum ion, the H-O bond becomes more polar. This means that the water molecule is more readily able to form hydrogen ions, which makes the solution acidic.
C)False!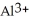 ions tend to make aqueous solutions basic since they pull the OH⁻ ion from the water molecule.
ions tend to make aqueous solutions basic since they pull the OH⁻ ion from the water molecule.
D)The formation of the oxide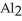
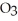 releases hydrogen ions to the aqueous solution making it acidic.
releases hydrogen ions to the aqueous solution making it acidic.
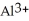 , usually acidic?
, usually acidic?A)The highly charged
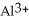 ion strips hydrogen ions away from water as if forms
ion strips hydrogen ions away from water as if forms 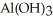 and releases them into the solution causing the solution to become acidic.
and releases them into the solution causing the solution to become acidic.B)As the water molecule binds to the aluminum ion, the H-O bond becomes more polar. This means that the water molecule is more readily able to form hydrogen ions, which makes the solution acidic.
C)False!
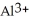 ions tend to make aqueous solutions basic since they pull the OH⁻ ion from the water molecule.
ions tend to make aqueous solutions basic since they pull the OH⁻ ion from the water molecule.D)The formation of the oxide
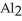
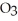 releases hydrogen ions to the aqueous solution making it acidic.
releases hydrogen ions to the aqueous solution making it acidic.
Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
43
Some molecules are able to stabilize a negative charge by passing it from one atom to the next by a flip-flopping of double bonds. This occurs when the negative charge is one atom away from an oxygen double bond as follows. Note that the curved arrows indicate the movement of electrons: 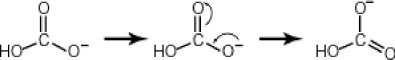 Why then is sulfuric acid so much stronger an acid than carbonic acid?
Why then is sulfuric acid so much stronger an acid than carbonic acid? 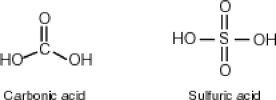
A)In sulfuric acid, the negative charge is able to flip-flop to two additional oxygens rather than only one as is the case for carbonic acid.
B)The two double bonded oxygens in
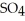 tend to destabilize the single bonded oxygens once the hydrogen ions form, thus making sulfuric acid more acidic.
tend to destabilize the single bonded oxygens once the hydrogen ions form, thus making sulfuric acid more acidic.
C)Since carbonic acid has resonance stabilization and sulfuric acid does not, sulfuric acid is less stable and more acidic.
D)The acid strength of two comparative molecules is directly proportional to the number of number of oxygens directly bonded to the central atom. Sulfuric acid, having four such oxygens, is more acidic.
 Why then is sulfuric acid so much stronger an acid than carbonic acid?
Why then is sulfuric acid so much stronger an acid than carbonic acid? 
A)In sulfuric acid, the negative charge is able to flip-flop to two additional oxygens rather than only one as is the case for carbonic acid.
B)The two double bonded oxygens in
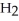
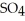 tend to destabilize the single bonded oxygens once the hydrogen ions form, thus making sulfuric acid more acidic.
tend to destabilize the single bonded oxygens once the hydrogen ions form, thus making sulfuric acid more acidic.C)Since carbonic acid has resonance stabilization and sulfuric acid does not, sulfuric acid is less stable and more acidic.
D)The acid strength of two comparative molecules is directly proportional to the number of number of oxygens directly bonded to the central atom. Sulfuric acid, having four such oxygens, is more acidic.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
44
Would it be easier or harder for a water molecule to donate a hydrogen ion if its polar H-O bond were even more polar? Why?
A)Easier. As polarity increases the bond classification tends towards ionic. An ionic bond would mean a greater separation of positive and negative charge making it easier for the water molecule to donate a hydrogen ion.
B)Harder. Increasing the polarity makes the bond stronger. The stronger the bond the more difficult it would be for the water molecule to donate a hydrogen ion.
C)Neither. Increasing the polarity of the O-H bond would not affect the hydrogen bonding between the water molecules and therefore have no effect on the water molecule's ability to donate a hydrogen ion.
D)Both. The ability of the water molecule to donate a hydrogen ion is dependent upon the environment you place it in. If the surrounding molecules are more covalent, donating the electron would be harder. However, if water found itself in a highly polar environment, increasing the O-H polarity would make it easier.
A)Easier. As polarity increases the bond classification tends towards ionic. An ionic bond would mean a greater separation of positive and negative charge making it easier for the water molecule to donate a hydrogen ion.
B)Harder. Increasing the polarity makes the bond stronger. The stronger the bond the more difficult it would be for the water molecule to donate a hydrogen ion.
C)Neither. Increasing the polarity of the O-H bond would not affect the hydrogen bonding between the water molecules and therefore have no effect on the water molecule's ability to donate a hydrogen ion.
D)Both. The ability of the water molecule to donate a hydrogen ion is dependent upon the environment you place it in. If the surrounding molecules are more covalent, donating the electron would be harder. However, if water found itself in a highly polar environment, increasing the O-H polarity would make it easier.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
45
Acetic acid, shown below, has 4 hydrogen atoms-one bonded to an oxygen and three bonded to a carbon. When this molecule behaves as an acid, it donates only the hydrogen bonded to the oxygen. The hydrogens bonded to the carbon remain intact. Why? 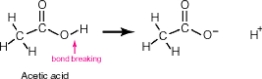
A)The oxygen is much better at accommodating a negative charge than is carbon.
B)The hydrogen attached to the oxygen extends farther away from the center of the molecule.
C)The carbon is bonded to three hydrogens while the oxygen is bonded to only one hydrogen.
D)The oxygen within acetic acid has two lone pairs of electrons that help to destabilize the oxygen-hydrogen bond.

A)The oxygen is much better at accommodating a negative charge than is carbon.
B)The hydrogen attached to the oxygen extends farther away from the center of the molecule.
C)The carbon is bonded to three hydrogens while the oxygen is bonded to only one hydrogen.
D)The oxygen within acetic acid has two lone pairs of electrons that help to destabilize the oxygen-hydrogen bond.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
46
Can an acid and a base react to form an acid?
A)No. Acids always react with bases to form salts.
B)Yes and No. The reaction can only occur if the substances reacting are true acids and bases.
C)Yes and No. The reaction can only occur if the reactants are organic/carboxylic acids and organic bases.
D)Yes. An acid and a base can react to form an acid if the acid is a strong acid and the base is a weak base.
A)No. Acids always react with bases to form salts.
B)Yes and No. The reaction can only occur if the substances reacting are true acids and bases.
C)Yes and No. The reaction can only occur if the reactants are organic/carboxylic acids and organic bases.
D)Yes. An acid and a base can react to form an acid if the acid is a strong acid and the base is a weak base.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
47
Sodium hydroxide, NaOH, is a very strong base. If a concentrated solution of this base were to spill on a latex glove you were wearing, it would feel like regular water. If the solution were to land directly on your skin, however, it would feel very slippery. Why?
A)The sodium hydroxide solution is reacting with your skin oils and transforming them into soap, which feels slippery.
B)The strong NaOH solution lifts the oil directly out of the skin cells and you feel the oil as a slippery sensation as it floats on the skin surface.
C)NaOH kills skin cells on contact. The dissolved skin cells feel slippery because the cells still contain the natural oils of the skin.
D)One of the properties of sodium hydroxide is that it is slippery. You are simply not able to sense the the slippery feel through a latex glove.
A)The sodium hydroxide solution is reacting with your skin oils and transforming them into soap, which feels slippery.
B)The strong NaOH solution lifts the oil directly out of the skin cells and you feel the oil as a slippery sensation as it floats on the skin surface.
C)NaOH kills skin cells on contact. The dissolved skin cells feel slippery because the cells still contain the natural oils of the skin.
D)One of the properties of sodium hydroxide is that it is slippery. You are simply not able to sense the the slippery feel through a latex glove.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following statements about strong and weak bases is not true?
A)A weak base does not completely dissociate in water.
B)A weak base will not react with a strong acid.
C)A strong base can be extremely corrosive.
D)A strong base will readily accept protons from even weak acids.
E)All of the above are untrue.
A)A weak base does not completely dissociate in water.
B)A weak base will not react with a strong acid.
C)A strong base can be extremely corrosive.
D)A strong base will readily accept protons from even weak acids.
E)All of the above are untrue.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
49
Sodium hydroxide, NaOH, is a strong base, which means that it readily accepts hydrogen ions. What products are formed when sodium hydroxide accepts a hydrogen ion from a water molecule?
A)water and sodium hydroxide
B)sodium hydroxide and hydronium ions
C)sodium ions and hydronium ions
D)sodium ions and water
A)water and sodium hydroxide
B)sodium hydroxide and hydronium ions
C)sodium ions and hydronium ions
D)sodium ions and water

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following statements about strong or weak bases is true?
A)A weak base completely separates into ions in water.
B)A weak base will not react with a strong acid.
C)A strong base is always corrosive.
D)A strong base will readily accept protons from even weak acids.
A)A weak base completely separates into ions in water.
B)A weak base will not react with a strong acid.
C)A strong base is always corrosive.
D)A strong base will readily accept protons from even weak acids.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the above images represents a solution of the strongest acid (HA)?
A)A
B)B
C)C
D)All of the above are strong acids.
E)None of the above are strong acids.
A)A
B)B
C)C
D)All of the above are strong acids.
E)None of the above are strong acids.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
52
A weak acid is added to a concentrated solution of hydrochloric acid. Does the solution become more or less acidic?
A)More acidic, since there are more hydronium ions being added to the solution.
B)Less acidic, since the solution becomes more dilute with a less concentrated solution of hydronium ions being added to the solution.
C)No change in acidity, since the concentration of the hydrochloric acid is too high to be changed by the weak solution.
D)Less acidic since the concentration of hydroxide ions will increase.
A)More acidic, since there are more hydronium ions being added to the solution.
B)Less acidic, since the solution becomes more dilute with a less concentrated solution of hydronium ions being added to the solution.
C)No change in acidity, since the concentration of the hydrochloric acid is too high to be changed by the weak solution.
D)Less acidic since the concentration of hydroxide ions will increase.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following statements about strong and weak acids is not true?
A)A weak acid is not as corrosive as a strong acid.
B)A weak acid does dissociate in water.
C)A strong acid will react with a strong base.
D)A weak acid will react with a strong base.
E)All of the above are untrue.
A)A weak acid is not as corrosive as a strong acid.
B)A weak acid does dissociate in water.
C)A strong acid will react with a strong base.
D)A weak acid will react with a strong base.
E)All of the above are untrue.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
54
What is the main characteristic of a strong acid?
A)It is completely dissociated in water.
B)It readily gives up its proton to a base.
C)It is corrosive.
D)It will damage your skin.
E)all of the above
A)It is completely dissociated in water.
B)It readily gives up its proton to a base.
C)It is corrosive.
D)It will damage your skin.
E)all of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
55
What is the main characteristic of a strong base?
A)It is completely dissociated in water.
B)It readily accepts an acidic proton.
C)It is corrosive.
D)It will damage your skin.
E)all of the above
A)It is completely dissociated in water.
B)It readily accepts an acidic proton.
C)It is corrosive.
D)It will damage your skin.
E)all of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
56
Which should be a stronger base: ammonia, 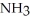 , or trifluoronitrogen,
, or trifluoronitrogen, 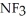 ? Why?
? Why? 
A)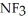 ; The fluorine atoms, being already highly electronegative, repel and tend to release the lone electron pair more easily, making it the stronger base.
; The fluorine atoms, being already highly electronegative, repel and tend to release the lone electron pair more easily, making it the stronger base.
B)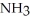 ; The fluorines have greater electronegativities and pull the lone pair electrons closer to nitrogen, thus making
; The fluorines have greater electronegativities and pull the lone pair electrons closer to nitrogen, thus making 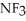 less willing to release the electron pair and thus be a weaker base.
less willing to release the electron pair and thus be a weaker base.
C)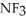 ; Ammonia tends to form a very pungent but weak base.
; Ammonia tends to form a very pungent but weak base. 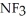 will definitely form a stronger base since the highly polar fluorines have replaced the weak hydrogens.
will definitely form a stronger base since the highly polar fluorines have replaced the weak hydrogens.
D)Neither; The nitrogens are large enough to shield the effect of either the hydrogens or fluorines from exerting any appreciable influence over the electron pair. Both molecules will have nearly identical base strength.
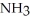 , or trifluoronitrogen,
, or trifluoronitrogen, 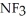 ? Why?
? Why? 
A)
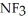 ; The fluorine atoms, being already highly electronegative, repel and tend to release the lone electron pair more easily, making it the stronger base.
; The fluorine atoms, being already highly electronegative, repel and tend to release the lone electron pair more easily, making it the stronger base.B)
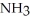 ; The fluorines have greater electronegativities and pull the lone pair electrons closer to nitrogen, thus making
; The fluorines have greater electronegativities and pull the lone pair electrons closer to nitrogen, thus making 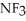 less willing to release the electron pair and thus be a weaker base.
less willing to release the electron pair and thus be a weaker base.C)
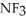 ; Ammonia tends to form a very pungent but weak base.
; Ammonia tends to form a very pungent but weak base. 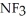 will definitely form a stronger base since the highly polar fluorines have replaced the weak hydrogens.
will definitely form a stronger base since the highly polar fluorines have replaced the weak hydrogens.D)Neither; The nitrogens are large enough to shield the effect of either the hydrogens or fluorines from exerting any appreciable influence over the electron pair. Both molecules will have nearly identical base strength.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
57
Why is phosphoric acid, 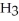
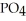 , a stronger acid than disodium hydrogen phosphate,
, a stronger acid than disodium hydrogen phosphate, 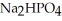 ?
? 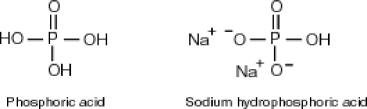
A)Phosphoric acid has three acidic hydrogens which makes it three times as acidic.
B)Some of the released sodium ions in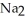 HP
HP 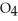 form NaOH (a base), which decreases the acidity of the
form NaOH (a base), which decreases the acidity of the 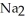 HP
HP 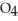 .
.
C)Phosphoric acid dissociates 100% in water whereas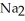 HP
HP 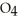 only dissociates about 50%.
only dissociates about 50%.
D)None of the above accurately describes why phosphoric acid is a stronger acid than disodium hydrogen phosphate.
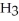
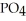 , a stronger acid than disodium hydrogen phosphate,
, a stronger acid than disodium hydrogen phosphate, 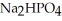 ?
? 
A)Phosphoric acid has three acidic hydrogens which makes it three times as acidic.
B)Some of the released sodium ions in
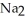 HP
HP 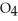 form NaOH (a base), which decreases the acidity of the
form NaOH (a base), which decreases the acidity of the 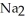 HP
HP 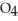 .
.C)Phosphoric acid dissociates 100% in water whereas
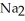 HP
HP 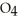 only dissociates about 50%.
only dissociates about 50%.D)None of the above accurately describes why phosphoric acid is a stronger acid than disodium hydrogen phosphate.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the above images would best describe a water solution of a weak acid (HA)the best?
A)A
B)B
C)C
D)All of the above are weak acids.
E)None of the above are weak acids.
A)A
B)B
C)C
D)All of the above are weak acids.
E)None of the above are weak acids.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
59
Arrange the following images of an aqueous base solution in order of increasing base strength: 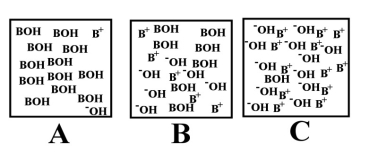
A)A, B, C
B)B, C, A
C)C, B, A
D)A, C, B
E)All are equally strong.

A)A, B, C
B)B, C, A
C)C, B, A
D)A, C, B
E)All are equally strong.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
60
Does a water molecule become more or less polar when bound to a metal ion, such as 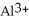 ? Why? (Hint: How might the electrons of the O-H covalent bonds in water "feel" about the highly charged aluminum ion?)
? Why? (Hint: How might the electrons of the O-H covalent bonds in water "feel" about the highly charged aluminum ion?)
A)Less polar. Aluminum ion is so highly polar that it attracts the electrons in a water molecule to itself and away from the oxygen atom in water, causing water to lose polar character.
B)More polar. The electrons in the O-H bond are drawn even closer to the oxygen (and away from the hydrogen)as they are drawn towards the positive charge of the aluminum ion.
C)Neither. Although polarity may increase as a water molecule approaches an aluminum ion, once the water molecule becomes bound to the metal ion its polarity reverts to its original status.
D)Both. As a first water molecule approaches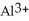 , there is an increase in water's polarity. However, the "bond" becomes less polar when the aluminum ion has captured all six water molecules to form
, there is an increase in water's polarity. However, the "bond" becomes less polar when the aluminum ion has captured all six water molecules to form 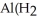 O
O 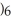 3+.
3+.
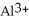 ? Why? (Hint: How might the electrons of the O-H covalent bonds in water "feel" about the highly charged aluminum ion?)
? Why? (Hint: How might the electrons of the O-H covalent bonds in water "feel" about the highly charged aluminum ion?)A)Less polar. Aluminum ion is so highly polar that it attracts the electrons in a water molecule to itself and away from the oxygen atom in water, causing water to lose polar character.
B)More polar. The electrons in the O-H bond are drawn even closer to the oxygen (and away from the hydrogen)as they are drawn towards the positive charge of the aluminum ion.
C)Neither. Although polarity may increase as a water molecule approaches an aluminum ion, once the water molecule becomes bound to the metal ion its polarity reverts to its original status.
D)Both. As a first water molecule approaches
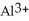 , there is an increase in water's polarity. However, the "bond" becomes less polar when the aluminum ion has captured all six water molecules to form
, there is an increase in water's polarity. However, the "bond" becomes less polar when the aluminum ion has captured all six water molecules to form 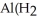 O
O 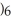 3+.
3+.
Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
61
What would the concentration of OH- be if the concentration of H3O+ was 1 × 10-5M? [H3O+] × [OH-] = Kw = 1 × 10-14
A)1 × 10-14
B)1 × 10-5
C)1 × 109
D)1 × 105
E)1 × 10-9
A)1 × 10-14
B)1 × 10-5
C)1 × 109
D)1 × 105
E)1 × 10-9

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
62
Which is the stronger acid: H-F or H-I?
A)H-F, because the electronegativity of fluorine is higher than that of iodine making the H-F bond more polar and more likely to release the hydrogen ion to solution.
B)H-F, because acid strength for binary acids decreases "down the group" of the periodic table.
C)H-I, because iodine releases the hydrogen in order to form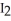 (s)at room temperature.
(s)at room temperature.
D)H-I, because the H-I bond is weaker and so it is easier to break to form hydrogen ions.
A)H-F, because the electronegativity of fluorine is higher than that of iodine making the H-F bond more polar and more likely to release the hydrogen ion to solution.
B)H-F, because acid strength for binary acids decreases "down the group" of the periodic table.
C)H-I, because iodine releases the hydrogen in order to form
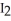 (s)at room temperature.
(s)at room temperature.D)H-I, because the H-I bond is weaker and so it is easier to break to form hydrogen ions.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
63
In behaving as both an acid and a base, water reacts with itself to form ________.
A)hydronium ions, H3O+.
B)hydroxide ions, OH-.
C)more water molecules.
D)two of the above.
E)all of the above.
A)hydronium ions, H3O+.
B)hydroxide ions, OH-.
C)more water molecules.
D)two of the above.
E)all of the above.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the above illustrations shows a neutral aqueous solution?
A)A
B)B
C)C
D)All of the above are neutral solutions.
E)none of the above
A)A
B)B
C)C
D)All of the above are neutral solutions.
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
65
Qualitatively, what happens to the hydroxide ion concentration if you decrease the hydronium ion concentration?
A)The concentration of hydroxide decreases to neutralize the excess hydronium.
B)The concentration of OH- stays the same but the ratio OH-/H3O+ changes.
C)The concentration of OH- increases but the ratio stays the same.
D)The concentration of OH- increases.
E)none of the above
A)The concentration of hydroxide decreases to neutralize the excess hydronium.
B)The concentration of OH- stays the same but the ratio OH-/H3O+ changes.
C)The concentration of OH- increases but the ratio stays the same.
D)The concentration of OH- increases.
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
66
Qualitatively, what happens to the hydronium ion concentration if you increase the hydroxide ion concentration?
A)The concentration of hydroxide decreases to neutralize the excess hydronium.
B)The concentration of H3O+ stays the same but the ratio changes.
C)The concentration of H3O+ increases but the ratio stays the same.
D)The concentration of H3O+ increases to neutralize the excess hydroxide.
E)none of the above
A)The concentration of hydroxide decreases to neutralize the excess hydronium.
B)The concentration of H3O+ stays the same but the ratio changes.
C)The concentration of H3O+ increases but the ratio stays the same.
D)The concentration of H3O+ increases to neutralize the excess hydroxide.
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
67
What is pH?
A)It is a numerical scale used to represent acidity or basicity.
B)It is the negative of the log of the hydronium ion concentration.
C)It is the negative of the log of the acidity of a solution.
D)It is the F sound.
E)both A and B
A)It is a numerical scale used to represent acidity or basicity.
B)It is the negative of the log of the hydronium ion concentration.
C)It is the negative of the log of the acidity of a solution.
D)It is the F sound.
E)both A and B

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
68
What would the concentration of OH- be if the concentration of H3O+ was 1 × 10-8M? [H3O+] × [OH-] = Kw = 1 × 10-14
A)1 × 10-14
B)1 × 10-6
C)1 × 108
D)1 × 106
E)1 × 10-8
A)1 × 10-14
B)1 × 10-6
C)1 × 108
D)1 × 106
E)1 × 10-8

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following statements describes a neutral solution?
A)[H3O+] > [OH-]
B)[H3O+] < [OH-]
C)[H3O+] × [OH-] ≠ 1 × 10-14
D)[H3O+]/[OH-] = 1 × 10-14
E)[H3O+]/[OH-] = 1
A)[H3O+] > [OH-]
B)[H3O+] < [OH-]
C)[H3O+] × [OH-] ≠ 1 × 10-14
D)[H3O+]/[OH-] = 1 × 10-14
E)[H3O+]/[OH-] = 1

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following statements describes a basic solution?
A)[H3O+] > [OH-]
B)[H3O+] < [OH-]
C)[H3O+] × [OH-] ≠ 1 × 10-14
D)[H3O+]/[OH-] = 1 × 10-14
E)[H3O+]/[OH-] = 1
A)[H3O+] > [OH-]
B)[H3O+] < [OH-]
C)[H3O+] × [OH-] ≠ 1 × 10-14
D)[H3O+]/[OH-] = 1 × 10-14
E)[H3O+]/[OH-] = 1

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
71
What would the concentration of H3O+ be if the concentration of OH- was 1 × 10-11M? [H3O+] × [OH-] = Kw = 1 × 10-14
A)1 × 10-3
B)1 × 103
C)1 × 1014
D)1 × 1012
E)1 × 10-6
A)1 × 10-3
B)1 × 103
C)1 × 1014
D)1 × 1012
E)1 × 10-6

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the above illustrations shows an acidic aqueous solution?
A)A
B)B
C)C
D)All of the above are neutral solutions.
E)none of the above
A)A
B)B
C)C
D)All of the above are neutral solutions.
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the above illustrations shows a basic aqueous solution?
A)A
B)B
C)C
D)All of the above are neutral solutions.
E)none of the above
A)A
B)B
C)C
D)All of the above are neutral solutions.
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following reactions illustrates an amphoteric compound?
A)2 HF ⇌ H2F+ + F-
B)NaOH + HBr ⇌ NaBr + H2O
C)2 H2 + O2 ⇌ 2 H2O
D)All are amphoteric.
E)none of the above
A)2 HF ⇌ H2F+ + F-
B)NaOH + HBr ⇌ NaBr + H2O
C)2 H2 + O2 ⇌ 2 H2O
D)All are amphoteric.
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
75
Why is the H-F bond so much stronger than the H-I bond?
A)H-F is a stronger bond because hydrogen and fluorine are more similar in nature than hydrogen and iodine. Both hydrogen and fluorine are diatomic and gases at room temperature.
B)H-F is stronger bond because iodine forms a solid at room temperature and fluorine forms a gas. Also, iodine tends to sublime at room temperature.
C)H-F is stronger because the fluorine atom is smaller and closer to the hydrogen atom causing greater attraction.
D)Actually, H-I is stronger because the electronegativity of the iodine atom is smaller that that of the fluorine atom.
A)H-F is a stronger bond because hydrogen and fluorine are more similar in nature than hydrogen and iodine. Both hydrogen and fluorine are diatomic and gases at room temperature.
B)H-F is stronger bond because iodine forms a solid at room temperature and fluorine forms a gas. Also, iodine tends to sublime at room temperature.
C)H-F is stronger because the fluorine atom is smaller and closer to the hydrogen atom causing greater attraction.
D)Actually, H-I is stronger because the electronegativity of the iodine atom is smaller that that of the fluorine atom.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
76
What do the brackets in the following equation represent? [H3O+] × [OH-] = Kw
A)The brackets mean the molarity of the compound inside them.
B)The molecule inside does not react with the other molecules in brackets.
C)The molecule in brackets is undergoing hydrogen bonding with the other molecule in brackets.
D)The first molecule in brackets is an acid and it reacts with the second molecule in brackets, which is a base.
E)none of the above
A)The brackets mean the molarity of the compound inside them.
B)The molecule inside does not react with the other molecules in brackets.
C)The molecule in brackets is undergoing hydrogen bonding with the other molecule in brackets.
D)The first molecule in brackets is an acid and it reacts with the second molecule in brackets, which is a base.
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
77
As the hydronium ion concentration increases, the pH ________.
A)goes down
B)gets larger
C)starts to affect the OH- concentration
D)starts to alter the chemical properties of the water molecules
E)stays constant
A)goes down
B)gets larger
C)starts to affect the OH- concentration
D)starts to alter the chemical properties of the water molecules
E)stays constant

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
78
How does a base like NH3 raise the hydroxide ion concentration?
A)The NH3 molecule removes a proton from a water molecule.
B)The NH3 molecule releases a proton that has to be neutralized by a water molecule.
C)The NH3 molecule is not a base and therefore does not change the hydroxide ion concentration.
D)The NH3 molecule releases reacts with a hydronium molecule, altering the number of hydroxide ions in the process.
E)none of the above
A)The NH3 molecule removes a proton from a water molecule.
B)The NH3 molecule releases a proton that has to be neutralized by a water molecule.
C)The NH3 molecule is not a base and therefore does not change the hydroxide ion concentration.
D)The NH3 molecule releases reacts with a hydronium molecule, altering the number of hydroxide ions in the process.
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
79
What would the concentration of H3O+ be if the concentration of OH- was 1 × 10-1M? [H3O+] × [OH-] = Kw = 1 × 10-14
A)1 × 10-3
B)1 × 10-2
C)1 × 10-4
D)1 × 1013
E)none of the above
A)1 × 10-3
B)1 × 10-2
C)1 × 10-4
D)1 × 1013
E)none of the above

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following statements describes an acidic solution?
A)[H3O+] > [OH-]
B)[H3O+] < [OH-]
C)[H3O+] × [OH-] ≠ 1 × 10-14
D)[H3O+]/[OH-] = 1 × 10-14
E)[H3O+]/[OH-] = 1
A)[H3O+] > [OH-]
B)[H3O+] < [OH-]
C)[H3O+] × [OH-] ≠ 1 × 10-14
D)[H3O+]/[OH-] = 1 × 10-14
E)[H3O+]/[OH-] = 1

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck


