Exam 10: Acids and Bases in Our Environment
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds162 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
As the pH increases, the hydroxide ion concentration ________.
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
B
Why do we use the pH scale to indicate the acidity of a solution rather than simply stating the concentration of hydronium ions?
Free
(Multiple Choice)
4.9/5  (30)
(30)
Correct Answer:
D
What would be the concentration of hydronium ions in a solution that had a pH = -3? Why would such a solution be impossible to prepare?
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
D
Which of the following statements describes a neutral solution?
(Multiple Choice)
4.8/5  (40)
(40)
For the following acid-base reaction, identify what is formed in the space marked. H2SO4 + KOH ⇌ KHSO4 + ???
(Multiple Choice)
4.7/5  (41)
(41)
For the following acid-base reaction, identify what is formed in the space marked. HF + KOH ⇌ ???? + H2O
(Multiple Choice)
4.9/5  (40)
(40)
According to the following reaction, which molecule is acting as a base? H2O + NH3 → OH- + NH4+
(Multiple Choice)
4.7/5  (40)
(40)
According to the following reaction, which molecule is acting as an acid? H3O+ + HSO4- → H2O + H2SO4
(Multiple Choice)
4.9/5  (40)
(40)
In the following buffer system, what happens to the concentration of the highlighted molecule if you add acid in the form of H3O+? HF + NaF + H2O ⇌ F- + H3O+ + Na+
(Multiple Choice)
4.7/5  (28)
(28)
The amphoteric reaction between two water molecules is endothermic, which means that this reaction requires the input of energy in order to proceed: Energy + 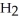 O +
O + 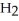 O →
O → 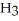
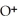 + O
+ O 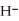 The warmer the temperature of the water, the more thermal energy is available for this reaction, and the more hydronium and hydroxide ions are formed. Based on this information, should the value of
The warmer the temperature of the water, the more thermal energy is available for this reaction, and the more hydronium and hydroxide ions are formed. Based on this information, should the value of 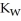 be expected to increase, decrease, or stay the same with increasing temperature?
be expected to increase, decrease, or stay the same with increasing temperature?
(Multiple Choice)
4.8/5  (45)
(45)
Would it be easier or harder for a water molecule to donate a hydrogen ion if its polar H-O bond were even more polar? Why?
(Multiple Choice)
4.9/5  (39)
(39)
What results when water, 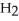 O, reacts with sulfur dioxide, S
O, reacts with sulfur dioxide, S 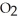 ?
?
(Multiple Choice)
4.9/5  (35)
(35)
What would be the best explanation for the fact that most natural water has a pH of about 5.6?
(Multiple Choice)
4.8/5  (37)
(37)
Sometimes an individual going through a traumatic experience cannot stop hyperventilating. In such a circumstance, it is recommended that the individual breath into a paper bag or cupped hands as a useful way to avoid an increase in blood pH, which can cause the person to pass out. Explain how this works.
(Multiple Choice)
4.7/5  (37)
(37)
A weak acid is added to a concentrated solution of hydrochloric acid. Does the solution become more or less acidic?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following statements about strong and weak bases is not true?
(Multiple Choice)
4.8/5  (34)
(34)
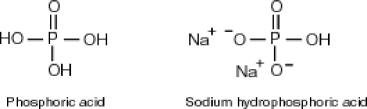
Why is phosphoric acid, 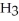
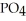 , a stronger acid than disodium hydrogen phosphate,
, a stronger acid than disodium hydrogen phosphate, 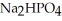 ?
? 
(Multiple Choice)
4.7/5  (27)
(27)
Cutting back on the pollutants that cause acid rain is one solution to the problem of acidified lakes. Suggest another.
(Multiple Choice)
4.9/5  (46)
(46)
Which of the following statements describes an acidic solution?
(Multiple Choice)
4.8/5  (28)
(28)
Showing 1 - 20 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)