Deck 13: Properties of Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/160
Play
Full screen (f)
Deck 13: Properties of Solutions
1
Hydration is a specific example of the phenomenon known generally as ________.
A)salutation
B)disordering
C)solvation
D)condensation
E)dilution
A)salutation
B)disordering
C)solvation
D)condensation
E)dilution
solvation
2
Which one of the following is most soluble in hexane (C6H14)?
A)CH3OH
B)CH3CH2CH2OH
C)CH3CH2OH
D)CH3CH2CH2CH2OH
E)CH3CH2CH2CH2CH2OH
A)CH3OH
B)CH3CH2CH2OH
C)CH3CH2OH
D)CH3CH2CH2CH2OH
E)CH3CH2CH2CH2CH2OH
CH3CH2CH2CH2CH2OH
3
Which one of the following substances is more likely to dissolve in benzene (C6H6)?
A)CH3CH2OH
B)NH3
C)NaCl
D)CCl4
E)HBr
A)CH3CH2OH
B)NH3
C)NaCl
D)CCl4
E)HBr
CCl4
4
Which of the following substances is more likely to dissolve in water?
A)HOCH2CH2OH
B)CHCl3
C) O CH3(CH2)9CH
CH3(CH2)9CH
D)CH3(CH2)8CH2OH
E)CCl4
A)HOCH2CH2OH
B)CHCl3
C) O
 CH3(CH2)9CH
CH3(CH2)9CHD)CH3(CH2)8CH2OH
E)CCl4

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
5
The phrase "like dissolves like" refers to the fact that ________.
A)gases can only dissolve other gases
B)polar solvents dissolve polar solutes and nonpolar solvents dissolve nonpolar solutes
C)solvents can only dissolve solutes of similar molar mass
D)condensed phases can only dissolve other condensed phases
E)polar solvents dissolve nonpolar solutes and vice versa
A)gases can only dissolve other gases
B)polar solvents dissolve polar solutes and nonpolar solvents dissolve nonpolar solutes
C)solvents can only dissolve solutes of similar molar mass
D)condensed phases can only dissolve other condensed phases
E)polar solvents dissolve nonpolar solutes and vice versa

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
6
The principal reason for the extremely low solubility of NaCl in benzene (C6H6)is the ________.
A)strong solvent-solvent interactions
B)hydrogen bonding in C6H6
C)strength of the covalent bond in NaCl
D)weak solvation of Na+ and Cl- by C6H6
E)increased disorder due to mixing of solute and solvent
A)strong solvent-solvent interactions
B)hydrogen bonding in C6H6
C)strength of the covalent bond in NaCl
D)weak solvation of Na+ and Cl- by C6H6
E)increased disorder due to mixing of solute and solvent

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
7
Compounds composed of a salt and water combined in definite proportions are known as ________.
A)clathrates
B)homogenates
C)ionic solids
D)molecular solids
E)hydrates
A)clathrates
B)homogenates
C)ionic solids
D)molecular solids
E)hydrates

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
8
A supersaturated solution ________.
A)is one with more than one solute
B)is one that has been heated
C)is one with a higher concentration than the solubility
D)must be in contact with undissolved solid
E)exists only in theory and cannot actually be prepared
A)is one with more than one solute
B)is one that has been heated
C)is one with a higher concentration than the solubility
D)must be in contact with undissolved solid
E)exists only in theory and cannot actually be prepared

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
9
A solution with a concentration higher than the solubility is ________.
A)not possible
B)unsaturated
C)supercritical
D)saturated
E)supersaturated
A)not possible
B)unsaturated
C)supercritical
D)saturated
E)supersaturated

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
10
In a saturated solution of a salt in water, ________.
A)the rate of crystallization > the rate of dissolution
B)the rate of dissolution > the rate of crystallization
C)seed crystal addition may cause massive crystallization
D)the rate of crystallization = the rate of dissolution
E)addition of more water causes massive crystallization
A)the rate of crystallization > the rate of dissolution
B)the rate of dissolution > the rate of crystallization
C)seed crystal addition may cause massive crystallization
D)the rate of crystallization = the rate of dissolution
E)addition of more water causes massive crystallization

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
11
Which one of the following vitamins is water soluble?
A)A
B)B
C)K
D)D
E)E
A)A
B)B
C)K
D)D
E)E

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
12
When solutions of strong electrolytes in water are formed, the ions are surrounded by water molecules. These interactions are described as a case of ________.
A)hydration
B)supersaturation
C)crystallization
D)dehydration
E)saturation
A)hydration
B)supersaturation
C)crystallization
D)dehydration
E)saturation

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
13
Which one of the following is least soluble in water?
A)CH3OH
B)CH3CH2CH2OH
C)CH3CH2OH
D)CH3CH2CH2CH2OH
E)CH3CH2CH2CH2CH2OH
A)CH3OH
B)CH3CH2CH2OH
C)CH3CH2OH
D)CH3CH2CH2CH2OH
E)CH3CH2CH2CH2CH2OH

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
14
When argon is placed in a container of neon, the argon spontaneously disperses throughout the neon because ________.
A)of the large attractive forces between argon and neon atoms
B)of hydrogen bonding
C)a decrease in energy occurs when the two mix
D)the dispersion of argon atoms produces an increase in disorder
E)of solvent-solute interactions
A)of the large attractive forces between argon and neon atoms
B)of hydrogen bonding
C)a decrease in energy occurs when the two mix
D)the dispersion of argon atoms produces an increase in disorder
E)of solvent-solute interactions

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
15
An unsaturated solution is one that ________.
A)has no double bonds
B)contains the maximum concentration of solute possible, and is in equilibrium with undissolved solute
C)has a concentration lower than the solubility
D)contains more dissolved solute than the solubility allows
E)contains no solute
A)has no double bonds
B)contains the maximum concentration of solute possible, and is in equilibrium with undissolved solute
C)has a concentration lower than the solubility
D)contains more dissolved solute than the solubility allows
E)contains no solute

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
16
Which one of the following substances is more likely to dissolve in CCl4?
A)CBr4
B)HBr
C)HCl
D)CH3CH2OH
E)NaCl
A)CBr4
B)HBr
C)HCl
D)CH3CH2OH
E)NaCl

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following substances is more likely to dissolve in CH3OH?
A)CCl4
B)Kr
C)N2
D)CH3CH2OH
E)H2
A)CCl4
B)Kr
C)N2
D)CH3CH2OH
E)H2

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
18
The dissolution of water in octane (C8H18)is prevented by ________.
A)London dispersion forces between octane molecules
B)hydrogen bonding between water molecules
C)dipole-dipole attraction between octane molecules
D)ion-dipole attraction between water and octane molecules
E)repulsion between like-charged water and octane molecules
A)London dispersion forces between octane molecules
B)hydrogen bonding between water molecules
C)dipole-dipole attraction between octane molecules
D)ion-dipole attraction between water and octane molecules
E)repulsion between like-charged water and octane molecules

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
19
Which one of the following is most soluble in water?
A)CH3OH
B)CH3CH2CH2OH
C)CH3CH2OH
D)CH3CH2CH2CH2OH
E)CH3CH2CH2CH2CH2OH
A)CH3OH
B)CH3CH2CH2OH
C)CH3CH2OH
D)CH3CH2CH2CH2OH
E)CH3CH2CH2CH2CH2OH

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following substances is least likely to dissolve in water?
A)HOCH2CH2OH
B)CHCl3
C) O CH3(CH2)9CH
CH3(CH2)9CH
D)CH3(CH2)8CH2OH
E)CCl4
A)HOCH2CH2OH
B)CHCl3
C) O
 CH3(CH2)9CH
CH3(CH2)9CHD)CH3(CH2)8CH2OH
E)CCl4

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
21
If the partial pressure of oxygen in the air a diver breathes is too great, ________.
A)respiratory tissue is damaged by oxidation
B)hyperventilation results
C)the urge to breathe is increased and excessive CO2 is removed from the body
D)the urge to breathe is reduced and not enough CO2 is removed from the body
E)No problems result from this situation.
A)respiratory tissue is damaged by oxidation
B)hyperventilation results
C)the urge to breathe is increased and excessive CO2 is removed from the body
D)the urge to breathe is reduced and not enough CO2 is removed from the body
E)No problems result from this situation.

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
22
The concentration of CO2 in a soft drink bottled with a partial pressure of CO2 of 6.5 atm over the liquid at 29 °C is 2.2 × 10-1 M. The Henry's law constant for CO2 at this temperature is ________.
A)2.2 × 10-1 mol/L-atm
B)7.6 × 10-3 mol/L-atm
C)5.6 × 10-3 mol/L-atm
D)3.4 × 10-2 mol/L-atm
E)More information is needed to solve the problem.
A)2.2 × 10-1 mol/L-atm
B)7.6 × 10-3 mol/L-atm
C)5.6 × 10-3 mol/L-atm
D)3.4 × 10-2 mol/L-atm
E)More information is needed to solve the problem.

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
23
A 0.100 m solution of which one of the following solutes will have the highest vapor pressure?
A)KClO4
B)Ca(ClO4)2
C)Al(ClO4)3
D)sucrose
E)NaCl
A)KClO4
B)Ca(ClO4)2
C)Al(ClO4)3
D)sucrose
E)NaCl

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following statements is false?
A)Nonpolar liquids tend to be insoluble in polar liquids.
B)The weaker the attraction between the solute and solvent molecules, the greater the solubility.
C)Substances with similar intermolecular attractive forces tend to be soluble in one another.
D)The solubility of a gas increases in direct proportion to its partial pressure above the solution.
E)The solubility of gases in water decreases with increasing temperature.
A)Nonpolar liquids tend to be insoluble in polar liquids.
B)The weaker the attraction between the solute and solvent molecules, the greater the solubility.
C)Substances with similar intermolecular attractive forces tend to be soluble in one another.
D)The solubility of a gas increases in direct proportion to its partial pressure above the solution.
E)The solubility of gases in water decreases with increasing temperature.

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
25
Calculate the molarity of a 17.5% (by mass)aqueous solution of nitric acid.
A)0.274 m
B)2.74 m
C)3.04 m
D)4.33 m
E)The density of the solution is needed to solve the problem.
A)0.274 m
B)2.74 m
C)3.04 m
D)4.33 m
E)The density of the solution is needed to solve the problem.

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
26
Pressure has an appreciable effect on the solubility of ________ in liquids.
A)gases
B)solids
C)liquids
D)salts
E)solids and liquids
A)gases
B)solids
C)liquids
D)salts
E)solids and liquids

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
27
A solution contains 11% by mass of sodium chloride. This means that ________.
A)there are 11 g of sodium chloride in in 1.0 mL of this solution
B)100 g of the solution contains 11 g of sodium chloride
C)100 mL of the solution contains 11 g of sodium chloride
D)the density of the solution is 11 g/mL
E)the molality of the solution is 11
A)there are 11 g of sodium chloride in in 1.0 mL of this solution
B)100 g of the solution contains 11 g of sodium chloride
C)100 mL of the solution contains 11 g of sodium chloride
D)the density of the solution is 11 g/mL
E)the molality of the solution is 11

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
28
A solution is prepared by dissolving calcium chloride in water and diluting to 500.0 mL. If this solution contains 44 ppm chloride ions, the concentration of calcium ions is ________ ppm.
A)44
B)88
C)22
D)11
E)500
A)44
B)88
C)22
D)11
E)500

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
29
As the concentration of a solute in a solution increases, the freezing point of the solution ________ and the vapor pressure of the solution ________.
A)increases, increases
B)increases, decreases
C)decreases, increases
D)decreases, decreases
E)decreases, is unaffected
A)increases, increases
B)increases, decreases
C)decreases, increases
D)decreases, decreases
E)decreases, is unaffected

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
30
The magnitudes of Kf and of Kb depend on the identity of the ________.
A)solute
B)solvent
C)solution
D)solvent and on temperature
E)solute and solvent
A)solute
B)solvent
C)solution
D)solvent and on temperature
E)solute and solvent

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
31
Of the concentration units below, only ________ uses kg of solvent in its calculation.
A)mass %
B)ppm
C)ppb
D)molarity
E)molality
A)mass %
B)ppm
C)ppb
D)molarity
E)molality

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
32
A 0.100 m solution of which one of the following solutes will have the lowest vapor pressure?
A)KClO4
B)Ca(ClO4)2
C)Al(ClO4)3
D)sucrose
E)NaCl
A)KClO4
B)Ca(ClO4)2
C)Al(ClO4)3
D)sucrose
E)NaCl

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
33
The concentration of CO2 in a soft drink bottled with a partial pressure of CO2 of 4.0 atm over the liquid at 25 °C is 1.2 × 10-1 M. The Henry's law constant for CO2 at this temperature is ________.
A)3.0 × 10-2 mol/L-atm
B)4.5 × 10-3 mol/L-atm
C)5.6 × 10-3 mol/L-atm
D)2.3 × 10-2 mol/L-atm
E)More information is needed to solve the problem.
A)3.0 × 10-2 mol/L-atm
B)4.5 × 10-3 mol/L-atm
C)5.6 × 10-3 mol/L-atm
D)2.3 × 10-2 mol/L-atm
E)More information is needed to solve the problem.

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
34
Calculate the molarity of a 10.0% (by mass)aqueous solution of hydrochloric acid.
A)0.274 m
B)2.74 m
C)3.04 m
D)4.33 m
E)The density of the solution is needed to solve the problem.
A)0.274 m
B)2.74 m
C)3.04 m
D)4.33 m
E)The density of the solution is needed to solve the problem.

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
35
Molality is defined as the ________.
A)moles solute/moles solvent
B)moles solute/liters solution
C)moles solute/kg solution
D)moles solute/kg solvent
E)none (dimensionless)
A)moles solute/moles solvent
B)moles solute/liters solution
C)moles solute/kg solution
D)moles solute/kg solvent
E)none (dimensionless)

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following choices has the compounds correctly arranged in order of increasing solubility in water? (least soluble to most soluble)
A)CCl4 < CHCl3 < NaNO3
B)CH3OH < CH4 < LiF
C)CH4 < NaNO3 < CHCl3
D)LiF < NaNO3 < CHCl3
E)CH3OH < Cl4 < CHCl3
A)CCl4 < CHCl3 < NaNO3
B)CH3OH < CH4 < LiF
C)CH4 < NaNO3 < CHCl3
D)LiF < NaNO3 < CHCl3
E)CH3OH < Cl4 < CHCl3

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
37
Which one of the following concentration units varies with temperature?
A)molarity
B)mass percent
C)mole fraction
D)molality
E)all of the above
A)molarity
B)mass percent
C)mole fraction
D)molality
E)all of the above

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
38
A solution contains 15 ppm of benzene. The density of the solution is 1.00 g/mL. This means that ________.
A)there are 15 mg of benzene in 1.0 g of this solution
B)100 g of the solution contains 15 g of benzene
C)1.0 g of the solution contains 15 × 10-6 g of benzene
D)1.0 L of the solution contains 15 g of benzene
E)the solution is 15% by mass of benzene
A)there are 15 mg of benzene in 1.0 g of this solution
B)100 g of the solution contains 15 g of benzene
C)1.0 g of the solution contains 15 × 10-6 g of benzene
D)1.0 L of the solution contains 15 g of benzene
E)the solution is 15% by mass of benzene

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
39
Which component of air is the primary problem in a condition known as "the bends"?
A)O2
B)CO2
C)He
D)N2
E)CO
A)O2
B)CO2
C)He
D)N2
E)CO

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
40
The solubility of nitrogen gas at 25 °C and 1 atm is 6.8 × 10-4 mol/L. If the partial pressure of nitrogen gas in air is 0.76 atm, what is the concentration (molarity)of dissolved nitrogen?
A)6.8 × 10-4 M
B)5.2 × 10-4 M
C)4.9 × 10-4 M
D)3.8 × 10-4 M
E)1.1 × 10-5 M
A)6.8 × 10-4 M
B)5.2 × 10-4 M
C)4.9 × 10-4 M
D)3.8 × 10-4 M
E)1.1 × 10-5 M

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
41
The most likely van't Hoff factor for an 0.01 m CaI2 solution is ________.
A)1.00
B)3.00
C)1.27
D)2.69
E)3.29
A)1.00
B)3.00
C)1.27
D)2.69
E)3.29

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
42
Pairs of liquids that will mix in all proportions are called ________ liquids.
A)miscible
B)unsaturated
C)polar liquids
D)saturated
E)supersaturated
A)miscible
B)unsaturated
C)polar liquids
D)saturated
E)supersaturated

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following liquids will have the lowest freezing point?
A)pure H2O
B)aqueous glucose (0.60 m)
C)aqueous sucrose (0.60 m)
D)aqueous FeI3 (0.24 m)
E)aqueous KF (0.50 m)
A)pure H2O
B)aqueous glucose (0.60 m)
C)aqueous sucrose (0.60 m)
D)aqueous FeI3 (0.24 m)
E)aqueous KF (0.50 m)

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following aqueous solutions will have the highest boiling point?
A)0.10 m Na2SO4
B)0.20 m glucose
C)0.25 m sucrose
D)0.10 m NaCl
E)0.10 m SrSO4
A)0.10 m Na2SO4
B)0.20 m glucose
C)0.25 m sucrose
D)0.10 m NaCl
E)0.10 m SrSO4

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
45
Colligative properties of solutions include all of the following except ________.
A)depression of vapor pressure upon addition of a solute to a solvent
B)elevation of the boiling point of a solution upon addition of a solute to a solvent
C)depression of the freezing point of a solution upon addition of a solute to a solvent
D)an increase in the osmotic pressure of a solution upon the addition of more solute
E)the increase of reaction rates with increase in temperature
A)depression of vapor pressure upon addition of a solute to a solvent
B)elevation of the boiling point of a solution upon addition of a solute to a solvent
C)depression of the freezing point of a solution upon addition of a solute to a solvent
D)an increase in the osmotic pressure of a solution upon the addition of more solute
E)the increase of reaction rates with increase in temperature

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
46
Hydrophobic colloids ________.
A)are those that contain water
B)can be stabilized by adsorption of ions
C)are those that do not contain water
D)can be stabilized by coagulation
E)will separate into two phases if they are stabilized
A)are those that contain water
B)can be stabilized by adsorption of ions
C)are those that do not contain water
D)can be stabilized by coagulation
E)will separate into two phases if they are stabilized

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
47
Calculate the vapor pressure of a solution made by dissolving 109 grams of glucose (molar mass = 180.2 g/mol)in 920.0 ml of water at 25 °C. The vapor pressure of pure water at 25 °C is 23.76 mm Hg. Assume the density of the solution is 1.00 g/ml.
A)0.278 mm Hg
B)0.605 mm Hg
C)22.98 mm Hg
D)23.48 mm Hg
E)23.76 mm Hg
A)0.278 mm Hg
B)0.605 mm Hg
C)22.98 mm Hg
D)23.48 mm Hg
E)23.76 mm Hg

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
48
A 1.35 m aqueous solution of compound X had a boiling point of 101.4 °C. Which one of the following could be compound X? The boiling point elevation constant for water is 0.52 °C/m.
A)CH3CH2OH
B)C6H12O6
C)Na3PO4
D)KCl
E)CaCl2
A)CH3CH2OH
B)C6H12O6
C)Na3PO4
D)KCl
E)CaCl2

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following cannot be a colloid?
A)an emulsion
B)an aerosol
C)a homogeneous mixture
D)a foam
E)All of the above are colloids.
A)an emulsion
B)an aerosol
C)a homogeneous mixture
D)a foam
E)All of the above are colloids.

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
50
Which one of the following solutes has a limiting van't Hoff factor (i)of 3 when dissolved in water?
A)KNO3
B)CH3OH
C)CCl4
D)Na2SO4
E)sucrose
A)KNO3
B)CH3OH
C)CCl4
D)Na2SO4
E)sucrose

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
51
The process of a substance sticking to the surface of another is called
A)absorption
B)diffusion
C)effusion
D)adsorption
E)coagulation
A)absorption
B)diffusion
C)effusion
D)adsorption
E)coagulation

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
52
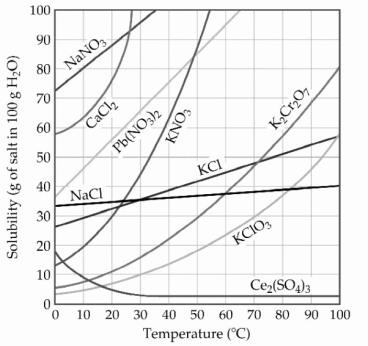
A sample of potassium nitrate (49.0 g)is dissolved in 101 g of water at 100 °C, with precautions taken to avoid evaporation of any water. The solution is cooled to 30.0 °C and no precipitate is observed. This solution is ________.
A)hydrated
B)placated
C)saturated
D)unsaturated
E)supersaturated

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
53
The ratio of the actual value of a colligative property to the value calculated, assuming the substance to be a nonelectrolyte, is referred to as ________.
A)Henry's law
B)vapor pressure lowering
C)the van't Hoff factor
D)freezing point depression
E)osmotic pressure
A)Henry's law
B)vapor pressure lowering
C)the van't Hoff factor
D)freezing point depression
E)osmotic pressure

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following liquids will have the highest freezing point?
A)pure H2O
B)aqueous glucose (0.60 m)
C)aqueous sucrose (0.60 m)
D)aqueous FeI3 (0.24 m)
E)aqueous KF (0.50 m)
A)pure H2O
B)aqueous glucose (0.60 m)
C)aqueous sucrose (0.60 m)
D)aqueous FeI3 (0.24 m)
E)aqueous KF (0.50 m)

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
55
Of the following, a 0.1 M aqueous solution of ________ will have the lowest freezing point.
A)NaCl
B)Al(NO3)3
C)K2CrO4
D)Na2SO4
E)sucrose
A)NaCl
B)Al(NO3)3
C)K2CrO4
D)Na2SO4
E)sucrose

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
56
The process of solute particles being surrounded by solvent particles is known as ________.
A)salutation
B)agglomeration
C)solvation
D)agglutination
E)dehydration
A)salutation
B)agglomeration
C)solvation
D)agglutination
E)dehydration

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
57
The solubility of Ar in water at 25 °C is 1.6 × 10-3 M when the pressure of the Ar above the solution is 1.0 atm. The solubility of Ar at a pressure of 2.5 atm is ________ M.
A)1.6 × 103
B)6.4 × 10-4
C)4.0 × 10-3
D)7.5 × 10-2
E)1.6 × 10-3
A)1.6 × 103
B)6.4 × 10-4
C)4.0 × 10-3
D)7.5 × 10-2
E)1.6 × 10-3

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following liquids will have the lowest freezing point?
A)pure H2O
B)aqueous glucose (0.050 m)
C)aqueous CoI2 (0.030 m)
D)aqueous FeI3 (0.030 m)
E)aqueous NaI (0.030 m)
A)pure H2O
B)aqueous glucose (0.050 m)
C)aqueous CoI2 (0.030 m)
D)aqueous FeI3 (0.030 m)
E)aqueous NaI (0.030 m)

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following liquids will have the highest freezing point?
A)pure H2O
B)aqueous glucose (0.050 m)
C)aqueous CoI2 (0.030 m)
D)aqueous FeI3 (0.030 m)
E)aqueous NaI (0.030 m)
A)pure H2O
B)aqueous glucose (0.050 m)
C)aqueous CoI2 (0.030 m)
D)aqueous FeI3 (0.030 m)
E)aqueous NaI (0.030 m)

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
60
Of the following, a 0.1 M aqueous solution of ________ will have the highest freezing point.
A)NaCl
B)Al(NO3)3
C)K2CrO4
D)Na2SO4
E)sucrose
A)NaCl
B)Al(NO3)3
C)K2CrO4
D)Na2SO4
E)sucrose

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
61

A sample of potassium chlorate (15.0 g)is dissolved in 201 g of water at 70 °C, with precautions taken to avoid evaporation of any water. The solution is cooled to 30.0 °C and no precipitate is observed. This solution is ________.
A)hydrated
B)miscible
C)saturated
D)unsaturated
E)supersaturated

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
62
The concentration of urea (MW = 60.0 g/mol)in a solution prepared by dissolving 16 g of urea in 39 g of H2O is ________ molal.
A)96
B)6.8
C)0.68
D)6.3
E)0.11
A)96
B)6.8
C)0.68
D)6.3
E)0.11

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
63
A solution is prepared by dissolving 23.7 g of CaCl2 in 375 g of water. The density of the resulting solution is 1.05 g/mL. The concentration of Cl- in this solution is ________ M.
A)0.214
B)0.562
C)1.12
D)1.20
E)6.64 × 10-2
A)0.214
B)0.562
C)1.12
D)1.20
E)6.64 × 10-2

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
64
What is the molarity of sodium chloride in solution that is 13.0% by mass sodium chloride and that has a density of 1.10 g/mL?
A)143
B)2.45
C)2.56
D)2.23
E)1.43 × 10-2
A)143
B)2.45
C)2.56
D)2.23
E)1.43 × 10-2

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
65
The vapor pressure of pure ethanol at 60 °C is 0.459 atm. Raoult's Law predicts that a solution prepared by dissolving 10.0 mmol naphthalene (nonvolatile)in 90.0 mmol ethanol will have a vapor pressure of ________ atm.
A)0.498
B)0.413
C)0.790
D)0.367
E)0.0918
A)0.498
B)0.413
C)0.790
D)0.367
E)0.0918

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
66
What is the molality of sodium chloride in solution that is 13.0% by mass sodium chloride and that has a density of 1.10 g/mL?
A)2.23
B)1.30
C)2.56
D)2.03
E)1.10
A)2.23
B)1.30
C)2.56
D)2.03
E)1.10

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
67
The mole fraction of HCl in a solution prepared by dissolving 5.5 g of HCl in 200 g of C2H6O is ________. The density of the solution is 0.79 g/mL.
A)0.027
B)0.034
C)0.028
D)0.035
E)0.151
A)0.027
B)0.034
C)0.028
D)0.035
E)0.151

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
68
A solution is prepared by dissolving 23.7 g of CaCl2 in 375 g of water. The density of the resulting solution is 1.05 g/mL. The concentration of CaCl2 in this solution is ________ molal.
A)0.214
B)0.569
C)5.70
D)63.2
E)1.76
A)0.214
B)0.569
C)5.70
D)63.2
E)1.76

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
69
A solution is prepared by dissolving 15.0 g of NH3 in 250.0 g of water. The density of the resulting solution is 0.974 g/mL. The molality of NH3 in the solution is ________ m.
A)0.00353
B)0.882
C)60.0
D)3.24
E)3.53
A)0.00353
B)0.882
C)60.0
D)3.24
E)3.53

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
70
A solution is prepared by dissolving 15.0 g of NH3 in 250.0 g of water. The density of the resulting solution is 0.974 g/mL. The molarity of NH3 in the solution is ________ M.
A)0.00353
B)0.882
C)60.0
D)3.24
E)3.53
A)0.00353
B)0.882
C)60.0
D)3.24
E)3.53

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
71
The concentration (M)of HCl in a solution prepared by dissolving 5.5 g of HCl in 200 g of C2H6O is ________ M. The density of the solution is 0.79 g/mL.
A)21
B)0.93
C)0.58
D)6.0 × 10-4
E)1.72
A)21
B)0.93
C)0.58
D)6.0 × 10-4
E)1.72

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
72
What is the mole fraction of sodium chloride in solution that is 13.0% by mass sodium chloride and that has a density of 1.10 g/mL?
A)0.0442
B)0.0462
C)0.223
D)0.483
E)0.505
A)0.0442
B)0.0462
C)0.223
D)0.483
E)0.505

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
73
The mole fraction of urea (MW = 60.0 g/mol)in a solution prepared by dissolving 16 g of urea in 39 g of H2O is ________.
A)0.58
B)0.37
C)0.13
D)0.11
E)9.1
A)0.58
B)0.37
C)0.13
D)0.11
E)9.1

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
74
A solution is prepared by dissolving 23.7 g of CaCl2 in 375 g of water. The density of the resulting solution is 1.05 g/mL. The concentration of CaCl2 in this solution is ________ molar.
A)0.564
B)0.571
C)0.569
D)0.537
E)0.214
A)0.564
B)0.571
C)0.569
D)0.537
E)0.214

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
75
The molarity of urea in a solution prepared by dissolving 16 g of urea (MW = 60.0 g/mol)in 39 g of H2O is ________ M. The density of the solution is 1.3 g/mL.
A)0.11
B)3.7
C)6.8
D)6.3
E)0.16
A)0.11
B)3.7
C)6.8
D)6.3
E)0.16

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
76

A sample of potassium nitrate (49.0 g)is dissolved in 101 g of water at 100 °C, with precautions taken to avoid evaporation of any water. The solution is cooled to 30.0 °C and a small amount of precipitate is observed. This solution is ________.
A)hydrated
B)placated
C)saturated
D)unsaturated
E)supersaturated

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
77

The solubility of MnSO4 monohydrate in water at 20 °C is 70.0 g per 100.0 mL of water. A solution at 20 °C that is 0.401 M in MnSO4 monohydrate is best described as a(n)________ solution. The formula weight of MnSO4 monohydrate is 168.97 g/mol.
A)hydrated
B)solvated
C)saturated
D)unsaturated
E)supersaturated

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
78

The solubility of MnSO4 monohydrate in water at 20 °C is 70.0 g per 100.0 mL of water. A solution at 20 °C that is 4.22 M in MnSO4 monohydrate is best described as a(n)________ solution. The formula weight of MnSO4 monohydrate is 168.97 g/mol.
A)hydrated
B)solvated
C)saturated
D)unsaturated
E)supersaturated

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
79
A solution is prepared by dissolving 23.7 g of CaCl2 in 375 g of water. The density of the resulting solution is 1.05 g/mL. The mole fraction of Cl- in this solution is ________.
A)0.0103
B)0.0200
C)0.0201
D)0.0632
E)0.0630
A)0.0103
B)0.0200
C)0.0201
D)0.0632
E)0.0630

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck
80
The concentration of HCl in a solution that is prepared by dissolving 5.5 g of HCl in 200 g of C2H6O is ________ molal.
A)27.5
B)7.5 × 10-4
C)3.3 × 10-2
D)0.75
E)1.3
A)27.5
B)7.5 × 10-4
C)3.3 × 10-2
D)0.75
E)1.3

Unlock Deck
Unlock for access to all 160 flashcards in this deck.
Unlock Deck
k this deck


