Exam 13: Properties of Solutions
Exam 1: Introduction: Matter and Measurement163 Questions
Exam 2: Atoms, Molecules, and Ions250 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations178 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry180 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding147 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry114 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
Which of the following choices has the compounds correctly arranged in order of increasing solubility in water? (least soluble to most soluble)
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
A
Which component of air is the primary problem in a condition known as "the bends"?
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
D
The dissolution of water in octane (C8H18)is prevented by ________.
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
B
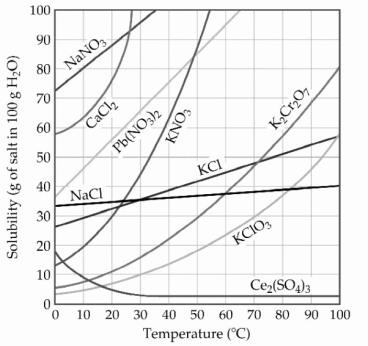 -The solubility of MnSO4 monohydrate in water at 20 °C is 70.0 g per 100.0 mL of water. A solution at 20 °C that is 0.401 M in MnSO4 monohydrate is best described as a(n)________ solution. The formula weight of MnSO4 monohydrate is 168.97 g/mol.
-The solubility of MnSO4 monohydrate in water at 20 °C is 70.0 g per 100.0 mL of water. A solution at 20 °C that is 0.401 M in MnSO4 monohydrate is best described as a(n)________ solution. The formula weight of MnSO4 monohydrate is 168.97 g/mol.
(Multiple Choice)
4.9/5  (47)
(47)
A 0.100 m solution of which one of the following solutes will have the highest vapor pressure?
(Multiple Choice)
4.7/5  (36)
(36)
 -The solubility of MnSO4 monohydrate in water at 20 °C is 70.0 g per 100.0 mL of water. A solution at 20 °C that is 4.22 M in MnSO4 monohydrate is best described as a(n)________ solution. The formula weight of MnSO4 monohydrate is 168.97 g/mol.
-The solubility of MnSO4 monohydrate in water at 20 °C is 70.0 g per 100.0 mL of water. A solution at 20 °C that is 4.22 M in MnSO4 monohydrate is best described as a(n)________ solution. The formula weight of MnSO4 monohydrate is 168.97 g/mol.
(Multiple Choice)
4.8/5  (42)
(42)
The principal reason for the extremely low solubility of NaCl in benzene (C6H6)is the ________.
(Multiple Choice)
4.9/5  (39)
(39)
Of the following, a 0.1 M aqueous solution of ________ will have the lowest freezing point.
(Multiple Choice)
4.9/5  (38)
(38)
Colligative properties of solutions include all of the following except ________.
(Multiple Choice)
4.9/5  (32)
(32)
A solution with a concentration higher than the solubility is ________.
(Multiple Choice)
4.9/5  (33)
(33)
A 1.35 m aqueous solution of compound X had a boiling point of 101.4 °C. Which one of the following could be compound X? The boiling point elevation constant for water is 0.52 °C/m.
(Multiple Choice)
4.9/5  (34)
(34)
Water (H2O)and the alcohol methanol (CH3OH)are infinitely soluble in each other. The primary intermolecular force responsible for this is ________.
(Short Answer)
4.8/5  (43)
(43)
Which of the following aqueous solutions will have the highest boiling point?
(Multiple Choice)
4.8/5  (38)
(38)
The solubility of nitrogen gas at 25 °C and 1 atm is 6.8 × 10-4 mol/L. If the partial pressure of nitrogen gas in air is 0.76 atm, what is the concentration (molarity)of dissolved nitrogen?
(Multiple Choice)
4.9/5  (34)
(34)
Calculate the molality of a 35.0% (by mass)aqueous solution of nitric acid.
(Multiple Choice)
4.7/5  (35)
(35)
A solution contains 33% phosphoric acid by mass. This means that ________.
(Multiple Choice)
4.9/5  (49)
(49)
The Henry's law constant for helium gas in water at 30 °C is 3.70 × 10-4 M/atm. When the partial pressure of helium above a sample of water is 0.400 atm, the concentration of helium in the water is ________ M.
(Multiple Choice)
4.9/5  (40)
(40)
When solutions of strong electrolytes in water are formed, the ions are surrounded by water molecules. These interactions are described as a case of ________.
(Multiple Choice)
4.9/5  (42)
(42)
A solution contains 39% phosphoric acid by mass. This means that ________.
(Multiple Choice)
4.9/5  (44)
(44)
Showing 1 - 20 of 160
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)