Deck 11: Enzymes: Biological Catalysts
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/24
Play
Full screen (f)
Deck 11: Enzymes: Biological Catalysts
1
An enzyme that catalyzes the intramolecular movement of a functional group from one carbon atom to another would be called a(n)____________.
A)isomerase
B)transferase
C)oxidoreductase
D)kinase
E)ligase
A)isomerase
B)transferase
C)oxidoreductase
D)kinase
E)ligase
A
2
Which amino acid most commonly serves as a general acid and general base in an enzyme mechanism?
A)serine
B)arginine
C)aspartic acid
D)histidine
E)cysteine
A)serine
B)arginine
C)aspartic acid
D)histidine
E)cysteine
D
3
What type of inhibitor would give the results seen in the following plot? 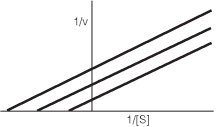
A)competitive inhibitor
B)mixed inhibitor
C)noncompetitive inhibitor
D)uncompetitive inhibitor
E)none of the above

A)competitive inhibitor
B)mixed inhibitor
C)noncompetitive inhibitor
D)uncompetitive inhibitor
E)none of the above
D
4
If an enzyme gave a rate enhancement of 3.4 * 107 over the noncatalyzed reaction, what is the G1 ‡ at 37 C?
A)5.3 kJ/mol
B)45 kJ/mol
C)9.5 kJ/mol
D)19 kJ/mol
E)none of the above
A)5.3 kJ/mol
B)45 kJ/mol
C)9.5 kJ/mol
D)19 kJ/mol
E)none of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following scenarios would result in a relatively low energy of activation?
A)an enzyme binds to the substrate with greater affinity than the transition state
B)an enzyme binds to both substrate and transition state with equal affinity
C)an enzyme binds to the transition state with greater affinity than the substrate
D)an enzyme binds only to the transition state
E)none of the above
A)an enzyme binds to the substrate with greater affinity than the transition state
B)an enzyme binds to both substrate and transition state with equal affinity
C)an enzyme binds to the transition state with greater affinity than the substrate
D)an enzyme binds only to the transition state
E)none of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following cofactors is used during the activation and transfer of carbon dioxide?
A)nicotinamide adenine dinucleotide (NAD)
B)thiamine pyrophosphate
C)biotin
D)coenzyme A
E)tetrahydrofolate
A)nicotinamide adenine dinucleotide (NAD)
B)thiamine pyrophosphate
C)biotin
D)coenzyme A
E)tetrahydrofolate

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
7
In the mechanism of chymotrypsin, which of the following amino acids found in the active site is correctly defined in terms of its role in the reaction?
A)serine: hydrogen bonds with carbonyl oxygen to withdraw electron density from the substrate
B)histidine: deprotonates aspartic acid to allow a nucleophilic attack to occur
C)aspartic acid: electrostatic stabilization of histidine to make a stronger base
D)cysteine: performs a nucleophilic attack on the carbonyl carbon of the substrate
E)none of the above
A)serine: hydrogen bonds with carbonyl oxygen to withdraw electron density from the substrate
B)histidine: deprotonates aspartic acid to allow a nucleophilic attack to occur
C)aspartic acid: electrostatic stabilization of histidine to make a stronger base
D)cysteine: performs a nucleophilic attack on the carbonyl carbon of the substrate
E)none of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
8
In the following enzyme reaction scheme, what sort of multi-enzyme kinetics are shown? 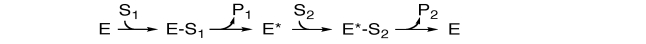
A)ordered substrate binding with random product release
B)ordered substrate binding with ordered product release
C)random substrate binding with ordered product release
D)random substrate binding with random product release
E)ping-pong mechanism
A)ordered substrate binding with random product release
B)ordered substrate binding with ordered product release
C)random substrate binding with ordered product release
D)random substrate binding with random product release
E)ping-pong mechanism

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
9
Since an enzyme is a catalyst, which of the following must be true?
A)an enzyme-catalyzed reaction is always exergonic
B)enzymes increase the rate of a reaction by providing a completely alternate mechanism to the uncatalyzed reaction
C)over the course of an enzyme-catalyzed reaction, the enzyme is not changed
D)in the absence of an enzyme, the reaction that is normally catalyzed by the enzyme will not occur
E)enzyme-catalyzed reactions never reach equilibrium
A)an enzyme-catalyzed reaction is always exergonic
B)enzymes increase the rate of a reaction by providing a completely alternate mechanism to the uncatalyzed reaction
C)over the course of an enzyme-catalyzed reaction, the enzyme is not changed
D)in the absence of an enzyme, the reaction that is normally catalyzed by the enzyme will not occur
E)enzyme-catalyzed reactions never reach equilibrium

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following explains why enzymes are extremely effective catalysts?
A)an enzyme stabilizes the transition state
B)enzymes bind very tightly to substrates
C)enzymes release products very rapidly
D)an enzyme can convert a normally endergonic reaction into an exergonic reaction
E)an enzyme lowers the energy of activation only for the forward reaction
A)an enzyme stabilizes the transition state
B)enzymes bind very tightly to substrates
C)enzymes release products very rapidly
D)an enzyme can convert a normally endergonic reaction into an exergonic reaction
E)an enzyme lowers the energy of activation only for the forward reaction

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
11
What must be true if KM is truly a measure of the affinity of enzyme and substrate?
A)kcat must be larger than KM
B)kcat must be smaller than KM
C)kcat must be about equal to k1
D)kcat must be much smaller than k-1
E)kcat must be much larger than k-1
A)kcat must be larger than KM
B)kcat must be smaller than KM
C)kcat must be about equal to k1
D)kcat must be much smaller than k-1
E)kcat must be much larger than k-1

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
12
Since the product of the reaction catalyzed by hexokinase, glucose-6-phosphate (G6P), can act as both a competitive and uncompetitive inhibitor, what can be said about the interaction between G6P and hexokinase?
A)G6P binds only to active site of the enzyme
B)G6P binds only to a regulatory site of the enzyme
C)G6P binds to both the active site and a regulatory site of the enzyme
D)G6P binds to one of the substrates, ATP, thus preventing ATP from binding to the active site
E)none of the above
A)G6P binds only to active site of the enzyme
B)G6P binds only to a regulatory site of the enzyme
C)G6P binds to both the active site and a regulatory site of the enzyme
D)G6P binds to one of the substrates, ATP, thus preventing ATP from binding to the active site
E)none of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
13
In a Lineweaver-Burke plot, what does the slope represent?
A)KM
B)Vmax
C)Vmax/KM
D)KM/Vmax
E)none of the above
A)KM
B)Vmax
C)Vmax/KM
D)KM/Vmax
E)none of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
14
Both ATP and CTP are allosteric regulators of the enzyme aspartate transcarbamoylase. Which of the following correctly identifies the activities of these two regulators?
A)ATP: activator; CTP: activator
B)ATP: activator; CTP: inhibitor
C)ATP: inhibitor; CTP: activator
D)ATP: inhibitor; CTP: inhibitor
E)none of the above
A)ATP: activator; CTP: activator
B)ATP: activator; CTP: inhibitor
C)ATP: inhibitor; CTP: activator
D)ATP: inhibitor; CTP: inhibitor
E)none of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
15
In noncompetitive inhibition, which of the following best explains how the inhibitor binds to the enzyme?
A)the inhibitor binds to the active site only after the substrate binds
B)the inhibitor binds to a site other than the active site only before the substrate binds
C)the inhibitor binds to a site other than the active site only after the substrate binds
D)the inhibitor binds to a site other than the active site either before or after the substrate binds
E)none of the above
A)the inhibitor binds to the active site only after the substrate binds
B)the inhibitor binds to a site other than the active site only before the substrate binds
C)the inhibitor binds to a site other than the active site only after the substrate binds
D)the inhibitor binds to a site other than the active site either before or after the substrate binds
E)none of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
16
The steady state assumption in enzyme kinetics:
A)insures that the product of an enzymatic reaction will always be formed
B)explains why enzymes are effective catalysts
C)states that the formation of ES is equal to its breakdown
D)is based upon the fact that the maximum velocity of an enzyme is very high
E)none of the above
A)insures that the product of an enzymatic reaction will always be formed
B)explains why enzymes are effective catalysts
C)states that the formation of ES is equal to its breakdown
D)is based upon the fact that the maximum velocity of an enzyme is very high
E)none of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
17
What are the expected changes in kinetics in the presence of a competitive inhibitor?
A)Vmax decreases, KM appears to decrease
B)Vmax does not change, KM appears to decrease
C)Vmax decreases, KM appears to increase
D)Vmax does not change, KM appears to increase
E)Vmax decreases, KM does not change
A)Vmax decreases, KM appears to decrease
B)Vmax does not change, KM appears to decrease
C)Vmax decreases, KM appears to increase
D)Vmax does not change, KM appears to increase
E)Vmax decreases, KM does not change

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is the most common form of reversible covalent modification for control of enzyme activity?
A)acetylation/deacetylation
B)adenylation/deadenylation
C)phosphorylation/dephosphorylation
D)ADP-ribosylation/de-ADP-ribosylation
E)none of the above
A)acetylation/deacetylation
B)adenylation/deadenylation
C)phosphorylation/dephosphorylation
D)ADP-ribosylation/de-ADP-ribosylation
E)none of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
19
The activity of lysozyme is greater than 50% of maximum between pH 3.8 and 6.1 with peak activity occurring around pH 5. Below 3.8 and above 6.1, the activity drops rapidly. Which of the following provides the best explanation for this?
A)two histidine residues in the active site must be protonated for enzyme activity
B)the active site contains an aspartic acid and glutamic acid, both of which must be deprotonated
C)the active site contains an aspartic acid that must be deprotonated and a glutamic acid that must be protonated
D)the active site contains a histidine that must be protonated and a glutamic acid that must be deprotonated
E)none of the above
A)two histidine residues in the active site must be protonated for enzyme activity
B)the active site contains an aspartic acid and glutamic acid, both of which must be deprotonated
C)the active site contains an aspartic acid that must be deprotonated and a glutamic acid that must be protonated
D)the active site contains a histidine that must be protonated and a glutamic acid that must be deprotonated
E)none of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
20
An enzymatic reaction has a Vmax of 100 M/min. At a substrate concentration of 5 M, the velocity is 25 M/min. What is the KM for the reaction?
A)5 M
B)10 M
C)15 M
D)20 M
E)none of the above
A)5 M
B)10 M
C)15 M
D)20 M
E)none of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
21
What term describes an inactive precursor of an enzyme such as the precursors to protease enzymes produced by the pancreas?
A)allosteric enzyme
B)zymogen
C)isozyme
D)ribozyme
E)hydrolase
A)allosteric enzyme
B)zymogen
C)isozyme
D)ribozyme
E)hydrolase

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
22
Below is a curve for the enzyme glycogen phosphorylase without any allosteric effectors present showing velocity as a function of the substrate, orthophosphate (Pi). Draw and label a curve that shows the result of addition of the allosteric activator, AMP. Draw and label a curve that shows the result of addition of the allosteric inhibitor, ATP. Glycogen phosphorylase is activated by phosphorylation. Which of these three curves most resembles what happens when glycogen phosphorylase is phosphorylated? 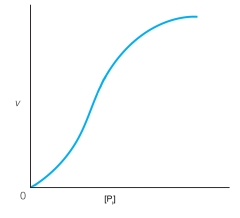


Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
23
Given the chymotrypsin catalytic triad and the peptide substrate, draw the first tetrahedral intermediate of the chymotrypsin mechanism. 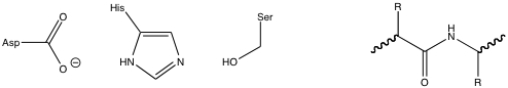


Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
24
A plot of enzyme activity with and without an inhibitor present gave the following plot. What type of inhibitor is present? How does this inhibitor function? What changes are seen in Vmax and KM? Draw a line that approximates the result from addition of twice as much inhibitor to the reaction. 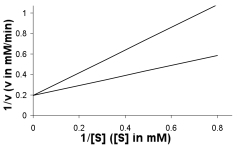


Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck


