Exam 11: Enzymes: Biological Catalysts
Exam 1: The Scope of Biochemistry17 Questions
Exam 2: The Matrix of Life: Weak Interactions in an Aqueous Environment25 Questions
Exam 3: The Energetics of Life25 Questions
Exam 4: Nucleic Acids28 Questions
Exam 5: Introduction to Proteins: the Primary Level of Protein Structure25 Questions
Exam 6: The Three-Dimensional Structure of Proteins24 Questions
Exam 7: Protein Function and Evolution27 Questions
Exam 8: Contractile Proteins and Molecular Motors19 Questions
Exam 9: Carbohydrates: Sugars, Saccharides, Glycans28 Questions
Exam 10: Lipids, Membranes and Cellular Transport25 Questions
Exam 11: Enzymes: Biological Catalysts24 Questions
Exam 12: Chemical Logic of Metabolism25 Questions
Exam 13: Carbohydrate Metabolism: Glycolysis, Gluconeogenesis, Glycogen Metabolism, and the Pentose Phosphate Pathway41 Questions
Exam 14: Citric Acid Cycle and Glyoxylate Cycle25 Questions
Exam 15: Electron Transport, Oxidative Phosphorylation, and Oxygen Metabolism24 Questions
Exam 16: Photosynthesis26 Questions
Exam 17: Lipid Metabolism I: Fatty Acids, Triacylglycerols, and Lipoproteins26 Questions
Exam 18: Interorgan and Intracellular Coordination of Energy Metabolism in Vertebrates22 Questions
Exam 19: Lipid Metabolism Ii: Membrane Lipids, Steroids, Isoprenoids, and Eicosanoids25 Questions
Exam 20: Metabolism of Nitrogenous Compounds I: Principles of Biosynthesis, Utilization, and Turnover25 Questions
Exam 21: Metabolism of Nitogenous Compounds II: Amino Acids, Porphyrins, and Neurotransmitters25 Questions
Exam 22: Nucleotide Metabolism25 Questions
Exam 23: Mechanisms of Signal Transduction24 Questions
Exam 24: Genes, Genomes and Chromosomes25 Questions
Exam 25: DNA Replication25 Questions
Exam 26: DNA Restructuring: Repair, Recombination, Rearrangement, Amplification25 Questions
Exam 27: Information Readout: Transcription and Post-Transcriptional Processing25 Questions
Exam 28: Information Decoding: Translation and Post-Translational Protein Processing28 Questions
Exam 29: Regulation of Gene Expression25 Questions
Select questions type
In the mechanism of chymotrypsin, which of the following amino acids found in the active site is correctly defined in terms of its role in the reaction?
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
C
In noncompetitive inhibition, which of the following best explains how the inhibitor binds to the enzyme?
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
D
The activity of lysozyme is greater than 50% of maximum between pH 3.8 and 6.1 with peak activity occurring around pH 5. Below 3.8 and above 6.1, the activity drops rapidly. Which of the following provides the best explanation for this?
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
C
A plot of enzyme activity with and without an inhibitor present gave the following plot. What type of inhibitor is present? How does this inhibitor function? What changes are seen in Vmax and KM? Draw a line that approximates the result from addition of twice as much inhibitor to the reaction. 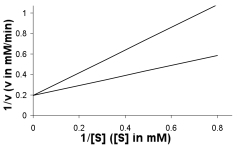
(Essay)
4.9/5  (39)
(39)
Which of the following is the most common form of reversible covalent modification for control of enzyme activity?
(Multiple Choice)
4.9/5  (34)
(34)
An enzymatic reaction has a Vmax of 100 M/min. At a substrate concentration of 5 M, the velocity is 25 M/min. What is the KM for the reaction?
(Multiple Choice)
5.0/5  (38)
(38)
Which of the following scenarios would result in a relatively low energy of activation?
(Multiple Choice)
4.9/5  (32)
(32)
Given the chymotrypsin catalytic triad and the peptide substrate, draw the first tetrahedral intermediate of the chymotrypsin mechanism. 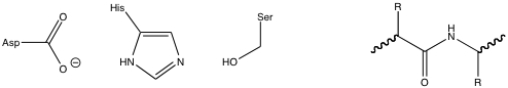
(Essay)
4.8/5  (42)
(42)
In the following enzyme reaction scheme, what sort of multi-enzyme kinetics are shown? 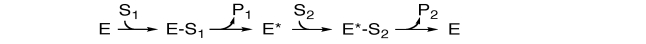
(Multiple Choice)
4.9/5  (36)
(36)
Below is a curve for the enzyme glycogen phosphorylase without any allosteric effectors present showing velocity as a function of the substrate, orthophosphate (Pi). Draw and label a curve that shows the result of addition of the allosteric activator, AMP. Draw and label a curve that shows the result of addition of the allosteric inhibitor, ATP. Glycogen phosphorylase is activated by phosphorylation. Which of these three curves most resembles what happens when glycogen phosphorylase is phosphorylated? 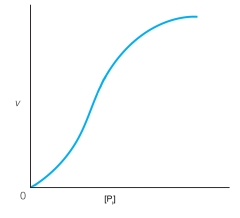
(Essay)
4.7/5  (35)
(35)
Which of the following explains why enzymes are extremely effective catalysts?
(Multiple Choice)
4.7/5  (34)
(34)
Which of the following cofactors is used during the activation and transfer of carbon dioxide?
(Multiple Choice)
4.8/5  (40)
(40)
Both ATP and CTP are allosteric regulators of the enzyme aspartate transcarbamoylase. Which of the following correctly identifies the activities of these two regulators?
(Multiple Choice)
4.7/5  (41)
(41)
Which amino acid most commonly serves as a general acid and general base in an enzyme mechanism?
(Multiple Choice)
4.8/5  (32)
(32)
What are the expected changes in kinetics in the presence of a competitive inhibitor?
(Multiple Choice)
4.8/5  (32)
(32)
Since the product of the reaction catalyzed by hexokinase, glucose-6-phosphate (G6P), can act as both a competitive and uncompetitive inhibitor, what can be said about the interaction between G6P and hexokinase?
(Multiple Choice)
4.9/5  (32)
(32)
What term describes an inactive precursor of an enzyme such as the precursors to protease enzymes produced by the pancreas?
(Multiple Choice)
4.8/5  (43)
(43)
What must be true if KM is truly a measure of the affinity of enzyme and substrate?
(Multiple Choice)
5.0/5  (39)
(39)
Showing 1 - 20 of 24
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)