Deck 15: Electron Transport, Oxidative Phosphorylation, and Oxygen Metabolism
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/24
Play
Full screen (f)
Deck 15: Electron Transport, Oxidative Phosphorylation, and Oxygen Metabolism
1
Which of the following describes a correct and complete electron transfer pathway?
A)NADH complex I CoQ complex III cyt c complex IV
B)NADH complex I complex II CoQ complex III cyt c complex IV
C)NADH complex I cyt c complex III CoQ complex IV
D)FADH2 CoQ complex II complex III cyt c complex IV
E)FADH2 complex II cyt c complex III CoQ complex IV
A)NADH complex I CoQ complex III cyt c complex IV
B)NADH complex I complex II CoQ complex III cyt c complex IV
C)NADH complex I cyt c complex III CoQ complex IV
D)FADH2 CoQ complex II complex III cyt c complex IV
E)FADH2 complex II cyt c complex III CoQ complex IV
NADH complex I CoQ complex III cyt c complex IV
2
What is the approximate P/O ratio if the source of electrons for the electron transport chain is FADH2?
A)1
B)1.5
C)2
D)2.5
E)3
A)1
B)1.5
C)2
D)2.5
E)3
B
3
The presence of 10 copies of the c subunit of the F0 complex of yeast ATP synthase allows for the synthesis of 1 ATP for every ______ proton(s)that are translocated across the inner mitochondrial membrane.
A)10
B)4
C)3.3
D)2.5
E)1
A)10
B)4
C)3.3
D)2.5
E)1
C
4
__________ increases oxygen consumption while decreasing ATP synthesis, but ____________ decreases both oxygen consumption and ATP synthesis.
A)rotenone; valinomycin
B)azide; 2,4-dinitrophenol
C)antimycin A; nigericin
D)2,4-dinitrophenol; oligomycin
E)cyanide; valinomycin
A)rotenone; valinomycin
B)azide; 2,4-dinitrophenol
C)antimycin A; nigericin
D)2,4-dinitrophenol; oligomycin
E)cyanide; valinomycin

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
5
In addition to iron, what metal is found in certain cytochromes?
A)manganese
B)copper
C)chromium
D)cobalt
E)zinc
A)manganese
B)copper
C)chromium
D)cobalt
E)zinc

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
6
The inhibition by an unknown inhibitor of electron transport was found to be removed by a large concentration of phenazine methosulfate. Since phenazine methosulfate is known to accept electrons from cytochrome b of complex III, which of the following is most similar to the unknown inhibitor?
A)amytal
B)antimycin A
C)carbon monoxide
D)cyanide
E)rotenone
A)amytal
B)antimycin A
C)carbon monoxide
D)cyanide
E)rotenone

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following acts as an uncoupler of the mitochondrial proton gradient due to its action as an ionophore?
A)valinomycin
B)nigericin
C)trifluorocarbonylcyanide phenylhydrazone
D)2,4-dinitrophenol
E)cyanide
A)valinomycin
B)nigericin
C)trifluorocarbonylcyanide phenylhydrazone
D)2,4-dinitrophenol
E)cyanide

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
8
Based on the following information, what is the G for the oxidation of malate by NAD+? NAD+ + H+ + 2e-
 NADH E ' = -0.32 V
NADH E ' = -0.32 V
Oxaloacetate + 2H+ + 2e-
 malate E ' = -0.17 V
malate E ' = -0.17 V
A)-29 kJ/mol
B)29 kJ/mol
C)-95 kJ/mol
D)95 kJ/mol
E)none of the above
 NADH E ' = -0.32 V
NADH E ' = -0.32 VOxaloacetate + 2H+ + 2e-
 malate E ' = -0.17 V
malate E ' = -0.17 VA)-29 kJ/mol
B)29 kJ/mol
C)-95 kJ/mol
D)95 kJ/mol
E)none of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
9
Within the electron transport chain, complex _____ represents the entry point for electrons from NADH while complex _____ represents the entry point for electrons from FADH2.
A)I; III
B)II; III
C)I; II
D)II; I
E)IV; III
A)I; III
B)II; III
C)I; II
D)II; I
E)IV; III

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
10
In the malate/aspartate shuttle, glutamate is converted to _________ in the mitochondria while aspartate is converted to ___________ in the cytosol.
A)oxaloacetate; malate
B) -ketoglutarate; oxaloacetate
C)oxaloacetate; glutamate
D)aspartate; -ketoglutarate
E)malate; oxaloacetate
A)oxaloacetate; malate
B) -ketoglutarate; oxaloacetate
C)oxaloacetate; glutamate
D)aspartate; -ketoglutarate
E)malate; oxaloacetate

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
11
What is the approximate free energy for transporting a mole of protons from the matrix to the intermembrane space under normal conditions within the mitochondria?
A)+220 kJ/mol
B)+21 kJ/mol
C)-21 kJ/mol
D)-220 kJ/mol
E)none of the above
A)+220 kJ/mol
B)+21 kJ/mol
C)-21 kJ/mol
D)-220 kJ/mol
E)none of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following uses the energy of the proton gradient to drive transport of its substrates?
A)ADP/ATP carrier
B)phosphate translocase in symport mode
C)phosphate translocase in antiport mode
D)pyruvate transport system
E)all of the above
A)ADP/ATP carrier
B)phosphate translocase in symport mode
C)phosphate translocase in antiport mode
D)pyruvate transport system
E)all of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
13
If the following two reactions were coupled, what would be the oxidizing agent for the spontaneous reaction? NAD+ + H+ + 2e-
 NADH E ' = -0.32 V
NADH E ' = -0.32 V
Acetaldehyde + 2H+ + 2e-
 ethanol E ' = -0.20 V
ethanol E ' = -0.20 V
A)NAD+
B)acetaldehyde
C)NADH
D)ethanol
E)H+
 NADH E ' = -0.32 V
NADH E ' = -0.32 VAcetaldehyde + 2H+ + 2e-
 ethanol E ' = -0.20 V
ethanol E ' = -0.20 VA)NAD+
B)acetaldehyde
C)NADH
D)ethanol
E)H+

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
14
Which amino acid is complexed to iron-sulfur clusters to enable them to associate with proteins?
A)methionine
B)serine
C)lysine
D)tyrosine
E)cysteine
A)methionine
B)serine
C)lysine
D)tyrosine
E)cysteine

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
15
The oxidized form of coenzyme Q is considered a _______ while the reduced form is considered a _______.
A)semiquinone; quinone
B)quinone; semiquinone
C)quinone; hydroquinone
D)hydroquinone; quinone
E)semiquinone; hydroquinone
A)semiquinone; quinone
B)quinone; semiquinone
C)quinone; hydroquinone
D)hydroquinone; quinone
E)semiquinone; hydroquinone

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following steps in the catalytic cycle of the F1 component is the most energetically unfavorable?
A)binding of ADP
B)binding of Pi
C)formation of ATP from ADP and Pi
D)release of ATP
E)all of the above are equally energetically unfavorable
A)binding of ADP
B)binding of Pi
C)formation of ATP from ADP and Pi
D)release of ATP
E)all of the above are equally energetically unfavorable

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following correctly describes what happens in the first stage of the Q cycle, considering that one cytochrome c is reduced in the process?
A)one QH2 is oxidized to QH while another Q is reduced to QH2
B)one QH2 is reduced to QH while another QH is oxidized to Q
C)one QH2 is oxidized to QH
D)one QH2 is oxidized to Q while another Q is reduced to QH
E)none of the above
A)one QH2 is oxidized to QH while another Q is reduced to QH2
B)one QH2 is reduced to QH while another QH is oxidized to Q
C)one QH2 is oxidized to QH
D)one QH2 is oxidized to Q while another Q is reduced to QH
E)none of the above

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following pathways takes place primarily within the inner mitochondrial membrane?
A)fatty acid -oxidation
B)electron transport
C)glycolysis
D)citric acid cycle
E)pentose phosphate pathway
A)fatty acid -oxidation
B)electron transport
C)glycolysis
D)citric acid cycle
E)pentose phosphate pathway

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
19
Rotation of the ____ subunit causes the _____ subunit to change from tight conformation where ATP is produced to open conformation where ATP is released.
A) ;
B) ;
C) ;
D) ;
E) ;
A) ;
B) ;
C) ;
D) ;
E) ;

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following correctly indicates the number of protons pumped into the intermembrane space at each complex of electron transport for each FADH2 that is oxidized by the electron transport pathway?
A)II: 2 III: 4 IV: 2
B)II: 0 III: 2 IV: 4
C)II: 2 III: 2 IV: 2
D)II: 0 III: 4 IV: 2
E)II: 0 III: 2 IV: 2
A)II: 2 III: 4 IV: 2
B)II: 0 III: 2 IV: 4
C)II: 2 III: 2 IV: 2
D)II: 0 III: 4 IV: 2
E)II: 0 III: 2 IV: 2

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
21
A(n)_______ incorporates oxygen from O2 into its products while a(n)_______ uses another oxidizing agent to accomplish the oxidation of its substrate.
A)oxidase; dioxygenase
B)dehydrogenase; oxygenase
C)oxygenase; oxidase
D)dehydrogenase; oxidase
E)dioxygenase; oxygenase
A)oxidase; dioxygenase
B)dehydrogenase; oxygenase
C)oxygenase; oxidase
D)dehydrogenase; oxidase
E)dioxygenase; oxygenase

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
22
Using the structure of coenzyme Q, show one reaction to produce the semiquinone and the subsequent reaction to produce the hydroquinone forms of the molecule. (R represents the isoprenoid tail). 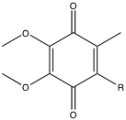


Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the reactive oxygen species is thought to damage lipid membranes?
A)semiquinone radical
B)superoxide
C)nitric oxide
D)hydrogen peroxide
E)hydroxyl radical
A)semiquinone radical
B)superoxide
C)nitric oxide
D)hydrogen peroxide
E)hydroxyl radical

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
24
Use the following half reactions to determine the reaction found in the pyruvate dehydrogenase complex. Calculate the G for the reaction.
lipoate + 2H+ + 2e-
 dihydrolipoate E ' = -0.29 V
dihydrolipoate E ' = -0.29 V
FAD + 2H+ + 2e-
 FADH2 E ' = -0.22 V
FADH2 E ' = -0.22 V
lipoate + 2H+ + 2e-
 dihydrolipoate E ' = -0.29 V
dihydrolipoate E ' = -0.29 VFAD + 2H+ + 2e-
 FADH2 E ' = -0.22 V
FADH2 E ' = -0.22 V 
Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck


