Deck 2: Acids and Bases: Central to Understanding Organic Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/37
Play
Full screen (f)
Deck 2: Acids and Bases: Central to Understanding Organic Chemistry
1
Which of the following is the strongest acid?
A)CH3CH2OH
B)CH3OCH3
C)CH3-NH-CH3
D)CH3-C CH
CH
E)CH3-CH CH2
CH2
A)CH3CH2OH
B)CH3OCH3
C)CH3-NH-CH3
D)CH3-C
 CH
CHE)CH3-CH
 CH2
CH2CH3CH2OH
2
Which of the following is not a conjugate acid-base pair?
A)"H2O, HO-"
B)"H2O, H3O+"
C)"HSO4-, H2SO4"
D)"-OH, O2-"
E)"NO3-, NO2-"
A)"H2O, HO-"
B)"H2O, H3O+"
C)"HSO4-, H2SO4"
D)"-OH, O2-"
E)"NO3-, NO2-"
"NO3-, NO2-"
3
Which of the following ions is the strongest acid?
A)H-
B)HO-
C)HSO4-
D)H2O
E)H3O+
A)H-
B)HO-
C)HSO4-
D)H2O
E)H3O+
H3O+
4
Which of the following is the strongest acid?
A)HF
B)H2O
C): NH3
D)CH4
E)CH3OH
A)HF
B)H2O
C): NH3
D)CH4
E)CH3OH

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
5
What is the conjugate acid of CH3NH2?
A)CH3NH3+
B)CH3NH-
C)NH4+
D)NH2-
A)CH3NH3+
B)CH3NH-
C)NH4+
D)NH2-

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
6
Which species act as bases in the following reaction? 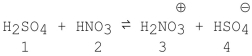
A)1 and 2
B)3 and 4
C)2 and 4
D)1 and 3
E)2 and 3
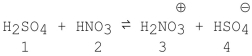
A)1 and 2
B)3 and 4
C)2 and 4
D)1 and 3
E)2 and 3

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
7
2-Propanol is shown below. Draw the structure of its conjugate base.
(CH3)2CHOH
(CH3)2CHOH

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
8
What is the pH of a 0.1 M solution of HCl? (Note: pKa for HCl is -6.)
A)6
B)-6
C)1
D)-8
E)-1
A)6
B)-6
C)1
D)-8
E)-1

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
9
Identify the compound with the highest pKa.
A)CH3CH3
B)HCCH
C)CH2CH2
D)CH3OH
E)CH3NH2
A)CH3CH3
B)HCCH
C)CH2CH2
D)CH3OH
E)CH3NH2

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
10
What is the conjugate base of CH3NH2?
A)CH3NH3+
B)CH3NH-
C)NH4+
D)NH2-
A)CH3NH3+
B)CH3NH-
C)NH4+
D)NH2-

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
11
Consider the set of compounds, NH3, HF, and H2O. Rank these compounds in order of increasing acidity and discuss your rationale.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is the strongest acid?
A)CH3OH
B)CH3OH2+
C)H2N-
D)CH3NH2
E)CH3NH3+
A)CH3OH
B)CH3OH2+
C)H2N-
D)CH3NH2
E)CH3NH3+

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
13
Give the conjugate acid and the conjugate base for HSO4-.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
14
If H2O has a pKa value of 15.7 and HF has a pKa value of 3.2, which is a stronger base, HO- or F-? Explain.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
15
Identify the compound with the highest pKa.
A)CH3NH2
B)CH3OH
C)CH3COOH
D)H2O
E)CH3NH3+
A)CH3NH2
B)CH3OH
C)CH3COOH
D)H2O
E)CH3NH3+

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
16
What is the product formed from the following acid-base reaction when ammonia functions as a base? The equilibrium lies far to the reactants. 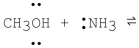
A)CH3O- + +NH4
B)CH2OH + +NH3
C)CH3OH2+ + -NH2
D)CH3NH2 + H2O
E)CH4 + NH2OH
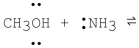
A)CH3O- + +NH4
B)CH2OH + +NH3
C)CH3OH2+ + -NH2
D)CH3NH2 + H2O
E)CH4 + NH2OH

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
17
What is the conjugate acid of NH3?
A)"+NH3"
B)"-NH"
C)"+NH4"
D)"-NH2"
E)"+NH2"
A)"+NH3"
B)"-NH"
C)"+NH4"
D)"-NH2"
E)"+NH2"

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
18
The pKa of CH3COOH is 4.8 and the pKa of HCOOH is 3.8. Given this information, one knows that ________.
A)CH3COOH completely ionizes in water
B)HCOOH is a weaker acid than CH3COOH
C)HCOO- is a weaker base than CH3COO-
D)CH3COOH reacts with HO- while HCOOH does not
E)HCOOH reacts with HO- while CH3COOH does not
A)CH3COOH completely ionizes in water
B)HCOOH is a weaker acid than CH3COOH
C)HCOO- is a weaker base than CH3COO-
D)CH3COOH reacts with HO- while HCOOH does not
E)HCOOH reacts with HO- while CH3COOH does not

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
19
The conjugate acid of H2O is ________.
A)H3O-
B)H3O
C)H3O+
D)HO-
E)H2O+
A)H3O-
B)H3O
C)H3O+
D)HO-
E)H2O+

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
20
Write a completed equation for the acid-base pair shown below.
HCO2H + -NH2 →
HCO2H + -NH2 →

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
21
The pKa of CH3COOH is 4.8. If the pH of an aqueous solution of CH3COOH and CH3COO- is 4.8, then one knows ________.
A)CH3COOH is completely ionized
B)[CH3COOH] > [CH3COO-]
C)[CH3COOH] = [CH3COO-]
D)[CH3COOH] < [CH3COO-]
E)CH3COOH is completely unionized
A)CH3COOH is completely ionized
B)[CH3COOH] > [CH3COO-]
C)[CH3COOH] = [CH3COO-]
D)[CH3COOH] < [CH3COO-]
E)CH3COOH is completely unionized

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
22
Buffering is used to maintain the pH of human blood in the relatively narrow 7.3 - 7.4 range. What acid/base pair serves to buffer the blood?
A)H2O / HO-
B)H3O+ / H2O
C)H2CO3 / HCO3-
D)NH4+ / NH3
E)HCl / Cl-
A)H2O / HO-
B)H3O+ / H2O
C)H2CO3 / HCO3-
D)NH4+ / NH3
E)HCl / Cl-

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
23
At what pH will the concentration of a compound with a pKa of 5.7 be 100 times greater in its acidic form than in its basic form?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
24
At what pH will 25% of a compound with a pKa of 5.3 be in its basic form?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
25
Explain why AlCl3 is a Lewis acid.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
26
Draw a resonance contributor and the resonance hybrid for HOCO2-.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
27
H-A is an acid with a pKa of 4.5. Which of the following statements about an aqueous solution of H-A is true?
A)At pH = 4.5, the solution contains much more H-A than A-
B)At pH = 4.5, the solution contains much more A- than H-A
C)At pH- 3.5, the solution contains about 90% A- and 10% H-A
D)At pH = 6.5, the soluton contains about 80% A- and 20% H-A
E)At pH = 5.5, the solution contains about 90% A- and 10% H-A
A)At pH = 4.5, the solution contains much more H-A than A-
B)At pH = 4.5, the solution contains much more A- than H-A
C)At pH- 3.5, the solution contains about 90% A- and 10% H-A
D)At pH = 6.5, the soluton contains about 80% A- and 20% H-A
E)At pH = 5.5, the solution contains about 90% A- and 10% H-A

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
28
The amino acid glycine (H3N+CH2CO2H)has two acidic Hs, one with pKa = 2.34 and the other with pKa=9.60. Draw the structure of the form of glycine that predominates at a pH of 12.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
29
HCN has a pKa = 9.1. What form of the compound, HCN or CN-, will predominate in a solution of pH = 7.0

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
30
Would you predict trifluoromethanesulfonic acid, CF3SO3H, to be a stronger or weaker acid than methanesulfonic acid, CH3SO3H? Explain your reasoning.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
31
Propanoic acid, CH3CH2COOH, has a pKa =4.9. Draw the structure of the conjugate base of propanoic acid and give the pH above which 90% of the compound will be in this conjugate base form.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
32
When a small amount of hexanoic acid [CH3(CH2)4CO2H, pKa~4.8], is added to a separatory funnel which contains the organic solvent diethyl ether and water with a pH of 2.0, it is found mainly in the ________ phase as ________.
A)ether; CH3(CH2)4CO2-
B)water; CH3(CH2)4CO2-
C)ether; CH3(CH2)4CO2H
D)water; CH3(CH2)4CO2H
E)none of the above
A)ether; CH3(CH2)4CO2-
B)water; CH3(CH2)4CO2-
C)ether; CH3(CH2)4CO2H
D)water; CH3(CH2)4CO2H
E)none of the above

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
33
Identify the most acidic carboxylic acid.
A)ICH2COOH
B)BrCH2COOH
C)CH3COOH
D)FCH2COOH
E)ClCH2COOH
A)ICH2COOH
B)BrCH2COOH
C)CH3COOH
D)FCH2COOH
E)ClCH2COOH

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is the strongest acid? 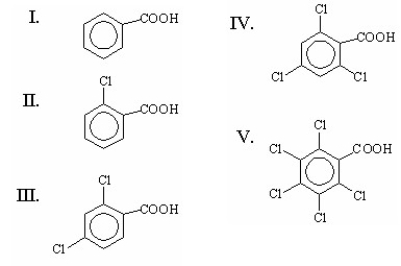
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
35
When a small amount of hexanoic acid [CH3(CH2)4CO2H, pKa~4.8], is added to a separatory funnel which contains the organic solvent diethyl ether and water with a pH of 12.0, it is found mainly in the ________ phase as ________.
A)ether; CH3(CH2)4CO2-
B)water; CH3(CH2)4CO2-
C)ether; CH3(CH2)4CO2H
D)water; CH3(CH2)4CO2H
E)none of the above
A)ether; CH3(CH2)4CO2-
B)water; CH3(CH2)4CO2-
C)ether; CH3(CH2)4CO2H
D)water; CH3(CH2)4CO2H
E)none of the above

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
36
Explain why : NF3 is a weaker base than : NH3.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following anions, CH3CHBrCO2- or CH3CHFCO2- is the stronger base? Explain your choice.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck


