Exam 2: Acids and Bases: Central to Understanding Organic Chemistry
Exam 1: Remembering General Chemistry: Electronic Structure and Bonding81 Questions
Exam 2: Acids and Bases: Central to Understanding Organic Chemistry37 Questions
Exam 3: An Introduction to Organic Compounds123 Questions
Exam 4: Isomers: the Arrangement of Atoms in Space111 Questions
Exam 5: Alkenes Thermodynamics and Kinetics76 Questions
Exam 6: Reactions of Alkenes93 Questions
Exam 7: Reactions of Alkynes Introduction to Multistep Synthesis119 Questions
Exam 8: Electron Delocalization, Resonance, and Aromaticity More About Molecular Orbital Theory166 Questions
Exam 9: Substitution Reactions of Alkyl Halides118 Questions
Exam 10: Elimination Reactions of Alkyl Halides Competition Between Substitution and Elimination94 Questions
Exam 11: Reactions of Alcohols, Ethers, Epoxides, and Sulfur-Containing Compounds100 Questions
Exam 12: Organometallic Compounds57 Questions
Exam 13: Reactions of Alkanes, Radicals130 Questions
Exam 14: Mass Spectrometry, Infrared Spectroscopy, and Uvvis Spectroscopy127 Questions
Exam 15: Nmr Spectroscopy110 Questions
Exam 16: Reactions of Carboxylic Acids and Carboxylic Acid Derivatives128 Questions
Exam 17: Reactions of Aldehydes and Ketones116 Questions
Exam 18: Reactions at the Alpha Carbon of Carbonyl Compounds113 Questions
Exam 19: Reactions of Benzene and Substituted Benzenes155 Questions
Exam 20: More About Amines Heterocylic Compounds115 Questions
Exam 21: Carbohydrates100 Questions
Exam 22: Amino Acids, Peptides, and Proteins105 Questions
Exam 23: Catalysis85 Questions
Exam 24: The Organic Mechanisms of the Coenzymes92 Questions
Exam 25: The Chemistry of Metabolism94 Questions
Exam 26: Nucleosides, Nucleotides, and Nucleic Acids85 Questions
Exam 27: Synthetic Polymers106 Questions
Exam 28: Pericyclic Reactions92 Questions
Select questions type
H-A is an acid with a pKa of 4.5. Which of the following statements about an aqueous solution of H-A is true?
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
E
Which of the following is the strongest acid? 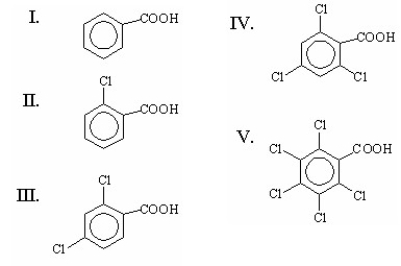
Free
(Multiple Choice)
4.9/5  (29)
(29)
Correct Answer:
E
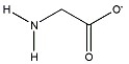
The amino acid glycine (H3N+CH2CO2H)has two acidic Hs, one with pKa = 2.34 and the other with pKa=9.60. Draw the structure of the form of glycine that predominates at a pH of 12.
Free
(Essay)
4.8/5  (28)
(28)
Correct Answer:
2-Propanol is shown below. Draw the structure of its conjugate base.
(CH3)2CHOH
(Essay)
4.8/5  (35)
(35)
What is the product formed from the following acid-base reaction when ammonia functions as a base? The equilibrium lies far to the reactants. 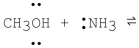
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following anions, CH3CHBrCO2- or CH3CHFCO2- is the stronger base? Explain your choice.
(Essay)
4.8/5  (42)
(42)
If H2O has a pKa value of 15.7 and HF has a pKa value of 3.2, which is a stronger base, HO- or F-? Explain.
(Essay)
4.8/5  (44)
(44)
Would you predict trifluoromethanesulfonic acid, CF3SO3H, to be a stronger or weaker acid than methanesulfonic acid, CH3SO3H? Explain your reasoning.
(Essay)
4.8/5  (39)
(39)
When a small amount of hexanoic acid [CH3(CH2)4CO2H, pKa~4.8], is added to a separatory funnel which contains the organic solvent diethyl ether and water with a pH of 2.0, it is found mainly in the ________ phase as ________.
(Multiple Choice)
4.9/5  (30)
(30)
At what pH will 25% of a compound with a pKa of 5.3 be in its basic form?
(Short Answer)
4.8/5  (38)
(38)
When a small amount of hexanoic acid [CH3(CH2)4CO2H, pKa~4.8], is added to a separatory funnel which contains the organic solvent diethyl ether and water with a pH of 12.0, it is found mainly in the ________ phase as ________.
(Multiple Choice)
4.9/5  (34)
(34)
The pKa of CH3COOH is 4.8 and the pKa of HCOOH is 3.8. Given this information, one knows that ________.
(Multiple Choice)
4.8/5  (38)
(38)
At what pH will the concentration of a compound with a pKa of 5.7 be 100 times greater in its acidic form than in its basic form?
(Short Answer)
4.8/5  (39)
(39)
Propanoic acid, CH3CH2COOH, has a pKa =4.9. Draw the structure of the conjugate base of propanoic acid and give the pH above which 90% of the compound will be in this conjugate base form.
(Essay)
4.9/5  (45)
(45)
Write a completed equation for the acid-base pair shown below.
HCO2H + -NH2 →
(Essay)
4.9/5  (41)
(41)
Showing 1 - 20 of 37
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)