Deck 8: Covalent Compounds: Bonding Theories and Molecular Structure
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/205
Play
Full screen (f)
Deck 8: Covalent Compounds: Bonding Theories and Molecular Structure
1
What is the smallest bond angle in SF6?
A)60°
B)90°
C)109.5°
D)120°
A)60°
B)90°
C)109.5°
D)120°
90°
2
What is the molecular geometry of SCl4?
A)seesaw
B)square planar
C)square pyramidal
D)tetrahedral
A)seesaw
B)square planar
C)square pyramidal
D)tetrahedral
seesaw
3
What is the angle between adjacent sp3 hybrid orbitals?
A)90°
B)109.5°
C)120°
D)180°
A)90°
B)109.5°
C)120°
D)180°
109.5°
4
The orbital hybridization on the central carbon atom in CH3CCH is
A)sp.
B)sp2.
C)sp3.
D)sp4.
A)sp.
B)sp2.
C)sp3.
D)sp4.

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
5
What is the F-B-F bond angle in BF3?
A)less than 109.5°
B)109.5°
C)120°
D)greater than 120°
A)less than 109.5°
B)109.5°
C)120°
D)greater than 120°

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following best describes CO2? It has a molecular geometry that is
A)linear molecular shape with no lone pairs on the I atom.
B)linear molecular shape with lone pairs on the I atom.
C)non-linear molecular shape with no lone pairs on the I atom.
D)non-linear molecular shape with lone pairs on the I atom.
A)linear molecular shape with no lone pairs on the I atom.
B)linear molecular shape with lone pairs on the I atom.
C)non-linear molecular shape with no lone pairs on the I atom.
D)non-linear molecular shape with lone pairs on the I atom.

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following should be nonlinear? 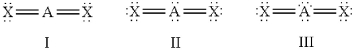
A)only I
B)only II
C)only III
D)II and III
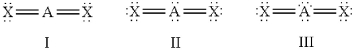
A)only I
B)only II
C)only III
D)II and III

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
8
A triple bond is generally composed of
A)three π bonds.
B)two π bonds and one σ bond.
C)one π bond and two σ bonds.
D)three σ bonds.
A)three π bonds.
B)two π bonds and one σ bond.
C)one π bond and two σ bonds.
D)three σ bonds.

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following should be nonplanar? 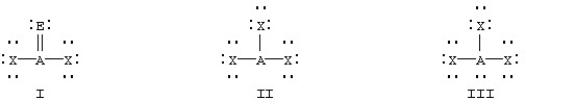
A)only I
B)only II
C)only III
D)I and III

A)only I
B)only II
C)only III
D)I and III

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
10
The C  O bond in COCl2 can be described as
O bond in COCl2 can be described as
A)a σ bond and a π bond,both involving sp hybrid orbitals on C.
B)a σ bond involving an sp hybrid orbital on C and a π bond involving a p orbital on C.
C)a σ bond and a π bond,both involving sp2 hybrid orbitals on C.
D)a σ bond involving an sp2 hybrid orbital on C and a π bond involving a p orbital on C.
 O bond in COCl2 can be described as
O bond in COCl2 can be described asA)a σ bond and a π bond,both involving sp hybrid orbitals on C.
B)a σ bond involving an sp hybrid orbital on C and a π bond involving a p orbital on C.
C)a σ bond and a π bond,both involving sp2 hybrid orbitals on C.
D)a σ bond involving an sp2 hybrid orbital on C and a π bond involving a p orbital on C.

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following would be expected to have sp2 hybridization on atom A? 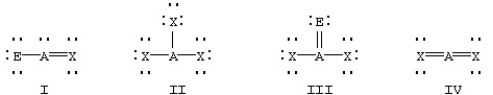
A)II
B)I and III
C)I,II,and III
D)I and IV
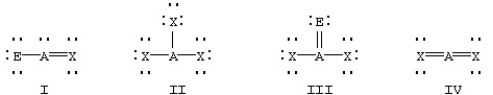
A)II
B)I and III
C)I,II,and III
D)I and IV

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
12
The number of sp2 hybrid orbitals on the carbon atom in CO32- is
A)one.
B)two.
C)three.
D)four.
A)one.
B)two.
C)three.
D)four.

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is not true?
A)The sp3 hybrid orbitals are degenerate.
B)An sp3 hybrid orbital may hold a lone pair of electrons.
C)An sp3 hybrid orbital may form a sigma bond by overlap with an orbital on another atom.
D)An sp3 hybrid orbital may form a pi bond by overlap with an orbital on another atom.
A)The sp3 hybrid orbitals are degenerate.
B)An sp3 hybrid orbital may hold a lone pair of electrons.
C)An sp3 hybrid orbital may form a sigma bond by overlap with an orbital on another atom.
D)An sp3 hybrid orbital may form a pi bond by overlap with an orbital on another atom.

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
14
Based on VSEPR theory,which should have the smallest XAX bond angle?
A)
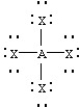
B)
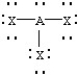
C)
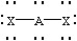
D)
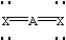
A)

B)

C)

D)
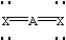

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
15
What is the molecular geometry of XeF4?
A)seesaw
B)square planar
C)square pyramidal
D)tetrahedral
A)seesaw
B)square planar
C)square pyramidal
D)tetrahedral

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is not a valence bond concept?
A)The greater the overlap between the orbitals on two atoms,the stronger the bond.
B)Lone pair electrons are in atomic orbitals or in hybrid atomic orbitals.
C)Atomic orbitals on two atoms may overlap to form antibonding orbitals.
D)A pair of electrons in a bond is shared by both atoms.
A)The greater the overlap between the orbitals on two atoms,the stronger the bond.
B)Lone pair electrons are in atomic orbitals or in hybrid atomic orbitals.
C)Atomic orbitals on two atoms may overlap to form antibonding orbitals.
D)A pair of electrons in a bond is shared by both atoms.

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
17
What is the molecular geometry of CH3-?
A)T-shaped
B)tetrahedral
C)trigonal planar
D)trigonal pyramidal
A)T-shaped
B)tetrahedral
C)trigonal planar
D)trigonal pyramidal

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
18
Which type of bond produces a charge cloud with a nodal plane containing the bond axis?
A)σ
B)π
C)σ and π
D)neither σ nor π
A)σ
B)π
C)σ and π
D)neither σ nor π

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
19
What is the molecular geometry of TeF5-?
A)octahedral
B)seesaw
C)square pyramidal
D)trigonal bipyramidal
A)octahedral
B)seesaw
C)square pyramidal
D)trigonal bipyramidal

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
20
Which orbital hybridization is associated with a tetrahedral cloud arrangement?
A)sp
B)sp2
C)sp3
D)sp4
A)sp
B)sp2
C)sp3
D)sp4

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
21
Which substance in each of the following pairs is expected to have the larger dispersion forces? 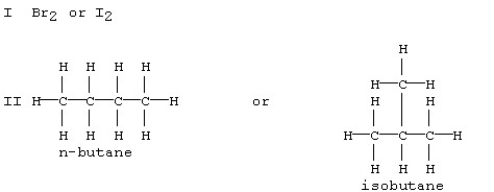
A)Br2 in set I and n-butane in set II
B)Br2 in set I and isobutane in set II
C)I2 in set I and n-butane in set II
D)I2 in set I and isobutane in set II

A)Br2 in set I and n-butane in set II
B)Br2 in set I and isobutane in set II
C)I2 in set I and n-butane in set II
D)I2 in set I and isobutane in set II

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
22
The MO diagram below is appropriate for B2.Based on this diagram,B2 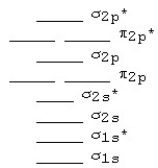
A)has a bond order of one and is diamagnetic.
B)has a bond order of one and is paramagnetic.
C)has a bond order of two and is diamagnetic.
D)has a bond order of two and is paramagnetic.

A)has a bond order of one and is diamagnetic.
B)has a bond order of one and is paramagnetic.
C)has a bond order of two and is diamagnetic.
D)has a bond order of two and is paramagnetic.

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following molecules will readily form hydrogen bones with H2O?
A)H2
B)C3H8
C)CH4
D)CH3OH
A)H2
B)C3H8
C)CH4
D)CH3OH

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
24
The HI bond has a length of 161 pm and 4.92% ionic character.What is the experimental dipole moment of HI?
A)0.380 D
B)0.772 D
C)3.80 D
D)7.72 D
A)0.380 D
B)0.772 D
C)3.80 D
D)7.72 D

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
25
Which compound below could have a zero dipole moment?
A)CCl2F2 (tetrahedral)
B)CuCl2F2 (tetrahedral)
C)PtCl2F2 (square planar)
D)SCl2F2 (see-saw)
A)CCl2F2 (tetrahedral)
B)CuCl2F2 (tetrahedral)
C)PtCl2F2 (square planar)
D)SCl2F2 (see-saw)

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
26
Which molecular orbital resembles a d-orbital?
A)σ
B)σ*
C)π
D)π*
A)σ
B)σ*
C)π
D)π*

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
27
Which molecular orbital resembles a p-orbital?
A)σ
B)σ*
C)π
D)π*
A)σ
B)σ*
C)π
D)π*

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
28
The following MO diagram is appropriate for Li2 and Be2.Based on this diagram, 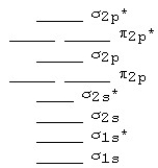
A)both are stable and diamagnetic.
B)Li2 is stable and diamagnetic,but Be2 is unstable.
C)Be2 is stable and diamagnetic,but Li2 is unstable.
D)Be2 is stable and paramagnetic,but Li2 is unstable.

A)both are stable and diamagnetic.
B)Li2 is stable and diamagnetic,but Be2 is unstable.
C)Be2 is stable and diamagnetic,but Li2 is unstable.
D)Be2 is stable and paramagnetic,but Li2 is unstable.

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
29
The dipole moment of BrF is 1.29 D,and its bond length is 178 pm.What is the percent ionic character of the Br-F bond?
A)3.9%
B)8.5%
C)15%
D)33%
A)3.9%
B)8.5%
C)15%
D)33%

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
30
What is the hybridization on the N atom in NO2- and in NO3-?
A)sp2 for NO2- and sp3 for NO3-
B)sp3 for NO2- and sp2 for NO3-
C)sp for NO2- and sp2 for NO3-
D)sp2 for both
A)sp2 for NO2- and sp3 for NO3-
B)sp3 for NO2- and sp2 for NO3-
C)sp for NO2- and sp2 for NO3-
D)sp2 for both

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
31
Which molecular orbitals for homonuclear diatomic molecules are degenerate?
A)π molecular orbitals
B)σ molecular orbitals
C)π molecular orbitals and σ molecular orbitals
D)neither π molecular orbitals nor σ molecular orbitals
A)π molecular orbitals
B)σ molecular orbitals
C)π molecular orbitals and σ molecular orbitals
D)neither π molecular orbitals nor σ molecular orbitals

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
32
AgCl is found to have 78.1% ionic character,and its gas phase dipole moment is 11.5 D.What is the distance between the Ag and Cl atoms in gaseous AgCl?
A)9.19 × 10-10 pm
B)14.7 pm
C)307 pm
D)903 pm
A)9.19 × 10-10 pm
B)14.7 pm
C)307 pm
D)903 pm

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following molecules has polar bonds but has no net dipole?
A)NH3
B)CCl4
C)CHCl3
D)CH4
A)NH3
B)CCl4
C)CHCl3
D)CH4

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
34
The bonds in the polyatomic ion CO32- are classified as
A)ionic.
B)metallic.
C)nonpolar covalent.
D)polar covalent.
A)ionic.
B)metallic.
C)nonpolar covalent.
D)polar covalent.

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following compounds exhibits hydrogen bonding?
A)CH3F
B)CH3OCH3
C)(CH3)3N
D)CH3CH2OH
A)CH3F
B)CH3OCH3
C)(CH3)3N
D)CH3CH2OH

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
36
The dipole moment of ClF is 0.88 D,and its bond length is 163 pm.What is the percent ionic character of the Cl-F bond?
A)0.54%
B)7.8%
C)11%
D)25%
A)0.54%
B)7.8%
C)11%
D)25%

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
37
If an electron is added to H2 it would go into a
A)σ molecular orbital and strengthen the H-H bond.
B)σ molecular orbital and weaken the H-H bond.
C)σ* molecular orbital and strengthen the H-H bond.
D)σ* molecular orbital and weaken the H-H bond.
A)σ molecular orbital and strengthen the H-H bond.
B)σ molecular orbital and weaken the H-H bond.
C)σ* molecular orbital and strengthen the H-H bond.
D)σ* molecular orbital and weaken the H-H bond.

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
38
Which atomic orbitals are involved in bonding and which as lone pair orbitals for N2H2? H - 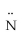 =
= 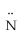 - H
- H
A)bonding: s on H,sp2 on N lone pair: p on N
B)bonding: sp2 on both H and N lone pair: p on N
C)bonding: s on H,p on N lone pair: sp2 on N
D)bonding: s on H,sp2 and p on N lone pair: sp2 on N
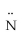 =
= 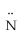 - H
- HA)bonding: s on H,sp2 on N lone pair: p on N
B)bonding: sp2 on both H and N lone pair: p on N
C)bonding: s on H,p on N lone pair: sp2 on N
D)bonding: s on H,sp2 and p on N lone pair: sp2 on N

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
39
A molecule with the formula XF3 where the element X has the hybridization sp3.Which of the following elements could be Y?
A)C
B)P
C)B
D)Si
A)C
B)P
C)B
D)Si

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
40
Compare the energies of molecular orbitals of homonuclear diatomic molecules with the energies of the atomic orbitals with which they correlate.
A)Both bonding and antibonding molecular orbitals lie lower in energy than the atomic orbitals.
B)Bonding orbitals are lower and antibonding orbitals are higher in energy than the atomic orbitals.
C)Bonding orbitals are higher and antibonding orbitals are lower in energy than the atomic orbitals.
D)Both bonding and antibonding molecular orbitals are higher in energy than the atomic orbitals.
A)Both bonding and antibonding molecular orbitals lie lower in energy than the atomic orbitals.
B)Bonding orbitals are lower and antibonding orbitals are higher in energy than the atomic orbitals.
C)Bonding orbitals are higher and antibonding orbitals are lower in energy than the atomic orbitals.
D)Both bonding and antibonding molecular orbitals are higher in energy than the atomic orbitals.

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
41
In which will the O-O bond be made stronger by removing an electron?
A)only O2
B)only O2-
C)only O22-
D)all of these
A)only O2
B)only O2-
C)only O22-
D)all of these

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
42
What is the geometry around the central atom in the following molecular model of NH4+? 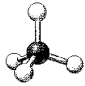
A)linear
B)bent
C)trigonal pyramidal
D)tetrahedral

A)linear
B)bent
C)trigonal pyramidal
D)tetrahedral

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
43
What is the geometry around the central atom in the following molecular model of SO2? 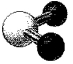
A)linear
B)bent
C)trigonal planar
D)trigonal pyramidal

A)linear
B)bent
C)trigonal planar
D)trigonal pyramidal

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
44
What is the geometry around the central atom in the following molecular model of BrF3? 
A)trigonal bipyramidal
B)seesaw
C)T-shaped
D)linear

A)trigonal bipyramidal
B)seesaw
C)T-shaped
D)linear

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
45
What is the geometry around the central atom in the following molecular model of NH2-? 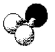
A)linear
B)bent
C)trigonal pyramidal
D)tetrahedral

A)linear
B)bent
C)trigonal pyramidal
D)tetrahedral

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
46
What is the geometry around the central atom in the following molecular model of XeF2? 
A)trigonal bipyramidal
B)seesaw
C)T-shaped
D)linear

A)trigonal bipyramidal
B)seesaw
C)T-shaped
D)linear

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
47
Which is the best description of the bonding in NO3-?
A)3 N-O σ bonds and no N-O π bonds
B)3 N-O σ bonds and 3 N-O π bonds
C)no N-O σ bonds and delocalized N-O π molecular orbitals extending over all four atoms
D)3 N-O σ bonds and delocalized N-O π molecular orbitals extending over all four atoms
A)3 N-O σ bonds and no N-O π bonds
B)3 N-O σ bonds and 3 N-O π bonds
C)no N-O σ bonds and delocalized N-O π molecular orbitals extending over all four atoms
D)3 N-O σ bonds and delocalized N-O π molecular orbitals extending over all four atoms

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
48
Given that O2 is paramagnetic and has a bond order of 2,and its highest occupied molecular orbital is antibonding,what would be the expected bond orders for O22- and O22+?
A)1 for O22- and 3 for O22+
B)3/2 for O22- and 5/2 for O22+
C)5/2 for O22- and 3/2 for O22+
D)3 for O22- and 1 for O22+
A)1 for O22- and 3 for O22+
B)3/2 for O22- and 5/2 for O22+
C)5/2 for O22- and 3/2 for O22+
D)3 for O22- and 1 for O22+

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
49
Use the following MO diagram for Be2,Be2+,and Be2-.Based on this diagram, 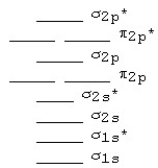
A)Be2+ is more stable than Be2,and Be2 is more stable than Be2-.
B)Be2- is more stable than Be2,and Be2 is more stable than Be2+.
C)Be2+ and Be2- are both more stable than Be2.
D)Be2 is more stable than either Be2+ or Be2-.

A)Be2+ is more stable than Be2,and Be2 is more stable than Be2-.
B)Be2- is more stable than Be2,and Be2 is more stable than Be2+.
C)Be2+ and Be2- are both more stable than Be2.
D)Be2 is more stable than either Be2+ or Be2-.

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
50
What is the geometry around the central atom in the following molecular model of SO32-? 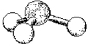
A)linear
B)bent
C)trigonal pyramidal
D)tetrahedral

A)linear
B)bent
C)trigonal pyramidal
D)tetrahedral

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
51
What is the geometry around the central atom in the following molecular model of SCl4? 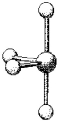
A)trigonal bipyramidal
B)seesaw
C)T-shaped
D)linear

A)trigonal bipyramidal
B)seesaw
C)T-shaped
D)linear

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
52
Based on molecular orbital theory,in order to weaken the N-N bond in N2,electrons should be
A)added.
B)removed.
C)either added or removed.
D)neither added nor removed.
A)added.
B)removed.
C)either added or removed.
D)neither added nor removed.

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
53
What is the geometry around the central atom in the following molecular model of NO3-? 
A)linear
B)bent
C)trigonal planar
D)trigonal pyramidal

A)linear
B)bent
C)trigonal planar
D)trigonal pyramidal

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
54
What is the geometry around the central atom in the following molecular model of H3O+? 
A)linear
B)bent
C)trigonal pyramidal
D)tetrahedral

A)linear
B)bent
C)trigonal pyramidal
D)tetrahedral

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
55
What is the geometry around the central atom in the following molecular model of NO2-? 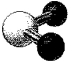
A)linear
B)bent
C)trigonal planar
D)trigonal pyramidal

A)linear
B)bent
C)trigonal planar
D)trigonal pyramidal

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
56
Which statement concerning any homonuclear diatomic molecule and its 1- ion must be true?
A)X2 must be more stable than X2-.
B)X2 must be less stable than X2-.
C)X2- must be paramagnetic and X2 must be diamagnetic.
D)X2- must be paramagnetic and X2 may be paramagnetic or diamagnetic.
A)X2 must be more stable than X2-.
B)X2 must be less stable than X2-.
C)X2- must be paramagnetic and X2 must be diamagnetic.
D)X2- must be paramagnetic and X2 may be paramagnetic or diamagnetic.

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
57
Which is paramagnetic?
A)N22+
B)N22-
C)O22+
D)O22-
A)N22+
B)N22-
C)O22+
D)O22-

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
58
The paramagnetism of O2 is explained by
A)coordinate covalent bonding.
B)molecular orbital theory.
C)resonance.
D)valence bond theory.
A)coordinate covalent bonding.
B)molecular orbital theory.
C)resonance.
D)valence bond theory.

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
59
Molecular orbitals extending over more than two atoms provide an explanation for
A)coordinate covalent bonding.
B)ionic bonding.
C)paramagnetism.
D)resonance.
A)coordinate covalent bonding.
B)ionic bonding.
C)paramagnetism.
D)resonance.

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
60
The SO3 molecule can be described as having 3 S-O σ bonds and π molecular orbitals containing
A)2 electrons.
B)4 electrons.
C)6 electrons.
D)8 electrons.
A)2 electrons.
B)4 electrons.
C)6 electrons.
D)8 electrons.

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
61
What is the geometry around the central atom in the following molecular model of XeF4? 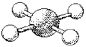
A)trigonal bipyramidal
B)octahedral
C)square pyramidal
D)square planar

A)trigonal bipyramidal
B)octahedral
C)square pyramidal
D)square planar

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
62
What is the bond angle in the following molecular model of I3-? 
A)less than 120°
B)120°
C)less than 180° but greater than 120°
D)180°

A)less than 120°
B)120°
C)less than 180° but greater than 120°
D)180°

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
63
What is the geometry around the central atom in the following molecular model of BrF5? 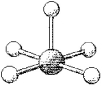
A)trigonal bipyramidal
B)octahedral
C)square pyramidal
D)square planar

A)trigonal bipyramidal
B)octahedral
C)square pyramidal
D)square planar

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
64
What are the bond angles in the following molecular model of TeF5-? 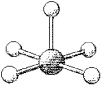
A)some less than 90° and some less than 120° but greater than 90°
B)90° and 120°
C)some less than 90° and some less than 180° but greater than 120°
D)90° and 180°

A)some less than 90° and some less than 120° but greater than 90°
B)90° and 120°
C)some less than 90° and some less than 180° but greater than 120°
D)90° and 180°

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
65
What are the bond angles in the following molecular model of NH4+? 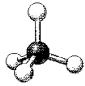
A)less than 109.5°
B)109.5°
C)less than 120° but greater than 109.5°
D)120°

A)less than 109.5°
B)109.5°
C)less than 120° but greater than 109.5°
D)120°

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
66
What are the bond angles in the following molecular model of BrF5? 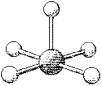
A)some less than 90° and some less than 120° but greater than 90°
B)90° and 120°
C)some less than 90° and some less than 180° but greater than 120°
D)90° and 180°

A)some less than 90° and some less than 120° but greater than 90°
B)90° and 120°
C)some less than 90° and some less than 180° but greater than 120°
D)90° and 180°

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
67
What are the bond angles in the following molecular model of PF6-? 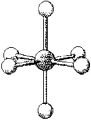
A)some less than 90° and some less than 120° but greater than 90°
B)90° and 120°
C)some less than 90° and some less than 180° but greater than 120°
D)90° and 180°

A)some less than 90° and some less than 120° but greater than 90°
B)90° and 120°
C)some less than 90° and some less than 180° but greater than 120°
D)90° and 180°

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
68
What are the bond angles in the following molecular model of H3O+? 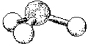
A)less than 109.5°
B)109.5°
C)less than 120° but greater than 109.5°
D)120°

A)less than 109.5°
B)109.5°
C)less than 120° but greater than 109.5°
D)120°

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
69
What are the bond angles in the following molecular model of BrF3? 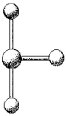
A)two less than 90° and one less than 180° but greater than 90°
B)90° and 180°
C)two less than 90° and one less than 120° but greater than 90°
D)90° and 120°

A)two less than 90° and one less than 180° but greater than 90°
B)90° and 180°
C)two less than 90° and one less than 120° but greater than 90°
D)90° and 120°

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
70
What is the geometry around the central atom in the following molecular model of ICl4-? 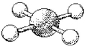
A)trigonal bipyramidal
B)octahedral
C)square pyramidal
D)square planar

A)trigonal bipyramidal
B)octahedral
C)square pyramidal
D)square planar

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
71
What is the bond angle in the following molecular model of NO2-? 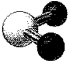
A)less than 109.5°
B)109.5°
C)less than 120° but greater than 109.5°
D)120°

A)less than 109.5°
B)109.5°
C)less than 120° but greater than 109.5°
D)120°

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
72
What is the bond angle in the following molecular model of SO2? 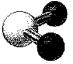
A)less than 109.5°
B)109.5°
C)less than 120° but greater than 109.5°
D)120°

A)less than 109.5°
B)109.5°
C)less than 120° but greater than 109.5°
D)120°

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
73
What are the bond angles in the following molecular model of SCl4? 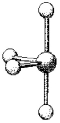
A)some less than 90° and one less than 180°
B)90° and 180°
C)some less than 90° one less than 120° but greater than 90°,and one less than 180° but greater than 120°
D)90°,120°,and 180°

A)some less than 90° and one less than 180°
B)90° and 180°
C)some less than 90° one less than 120° but greater than 90°,and one less than 180° but greater than 120°
D)90°,120°,and 180°

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
74
What is the bond angle in the following molecular model of XeF2? 
A)less than 120°
B)120°
C)less than 180° but greater than 120°
D)180°

A)less than 120°
B)120°
C)less than 180° but greater than 120°
D)180°

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
75
What are the bond angles in the following molecular model of NO3-? 
A)less than 109.5°
B)109.5°
C)less than 120° but greater than 109.5°
D)120°

A)less than 109.5°
B)109.5°
C)less than 120° but greater than 109.5°
D)120°

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
76
What are the bond angles in the following molecular model of XeF4? 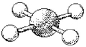
A)some less than 90° and some less than 120° but greater than 90°
B)90°
C)some less than 90° and some less than 180° but greater than 90°
D)90° and 180°

A)some less than 90° and some less than 120° but greater than 90°
B)90°
C)some less than 90° and some less than 180° but greater than 90°
D)90° and 180°

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
77
What is the geometry around the central atom in the following molecular model of TeF5-? 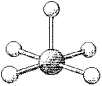
A)trigonal bipyramidal
B)octahedral
C)square pyramidal
D)square planar

A)trigonal bipyramidal
B)octahedral
C)square pyramidal
D)square planar

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
78
What is the bond angle in the following molecular model of NH2-? 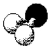
A)less than 109.5°
B)109.5°
C)less than 120° but greater than 109.5°
D)120°

A)less than 109.5°
B)109.5°
C)less than 120° but greater than 109.5°
D)120°

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
79
What is the geometry around the central atom in the following molecular model of I3-? 
A)trigonal bipyramidal
B)seesaw
C)T-shaped
D)linear

A)trigonal bipyramidal
B)seesaw
C)T-shaped
D)linear

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck
80
What are the bond angles in the following molecular model of SO32-? 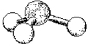
A)less than 109.5°
B)109.5°
C)less than 120° but greater than 109.5°
D)120°

A)less than 109.5°
B)109.5°
C)less than 120° but greater than 109.5°
D)120°

Unlock Deck
Unlock for access to all 205 flashcards in this deck.
Unlock Deck
k this deck


