Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
The dipole moment of BrF is 1.29 D,and its bond length is 178 pm.What is the percent ionic character of the Br-F bond?
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
C
Which of the following best describes CO2? It has a molecular geometry that is
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
A
What is the geometry around the central atom in the following molecular model of NH2-? 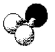
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
B
Based on molecular orbital theory,in order to weaken the N-N bond in N2,electrons should be
(Multiple Choice)
5.0/5  (36)
(36)
Molecular orbitals extending over more than two atoms provide an explanation for
(Multiple Choice)
4.9/5  (34)
(34)
Of BrF3 and PF3,the one with the smaller bond angles is ________.
(Short Answer)
4.7/5  (39)
(39)
What is the geometry around the central atom in the following molecular model of PF5? 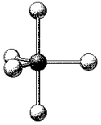
(Multiple Choice)
4.8/5  (44)
(44)
Predict whether removing two electrons from F2 will create an ion that is diamagnetic or paramagnetic and have an F-F that is stronger or weaker than the bond in F2.
(Short Answer)
4.7/5  (45)
(45)
What is the geometry around the central atom in the following molecular model of CCl4? 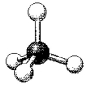
(Multiple Choice)
4.9/5  (39)
(39)
AgCl is found to have 78.1% ionic character,and its gas phase dipole moment is 11.5 D.What is the distance between the Ag and Cl atoms in gaseous AgCl?
(Multiple Choice)
5.0/5  (47)
(47)
Which of the following compounds exhibits only dispersion and dipole-dipole intermolecular interactions?
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following compounds exhibits hydrogen bonding?
(Multiple Choice)
4.9/5  (41)
(41)
The Br-Cl bond has 5.05% ionic character and a dipole moment of 0.518 D.What is the distance between atoms in BrCl?
(Short Answer)
4.9/5  (39)
(39)
According to molecular orbital theory,is the highest energy orbital that contains an electron antibonding or bonding in O22-?
(Short Answer)
4.8/5  (30)
(30)
What are the bond angles in the following molecular model of NH4+? 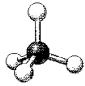
(Multiple Choice)
4.9/5  (34)
(34)
What is the geometry around the central atom in the following molecular model of NO3-? 
(Multiple Choice)
4.8/5  (31)
(31)
Showing 1 - 20 of 205
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)