Deck 12: Temperature and Heat
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/66
Play
Full screen (f)
Deck 12: Temperature and Heat
1
A steel string guitar is strung so that there is negligible tension in the strings at a temperature of 24.9 °C. The guitar is taken to an outdoor winter concert where the temperature of the strings decreases to -15.1 °C. The cross-sectional area of a particular string is 5.5 × 10-6 m2. The distance between the points where the string is attached does not change. For steel, Young's modulus is 2.0 × 1011 N/m2; and the coefficient of linear expansion is 1.2 × 10-5/C°. Use your knowledge of linear thermal expansion and stress to calculate the tension in the string at the concert.
A)530 N
B)240 N
C)120 N
D)60 N
E)30 N
A)530 N
B)240 N
C)120 N
D)60 N
E)30 N
530 N
2
Three thermometers are in the same water bath. After thermal equilibrium is established, it is found that the Celsius thermometer reads 0 °C, the Fahrenheit thermometer reads 12 °F, and the Kelvin thermometer reads 273 K. Which one of the following statements is the most reasonable conclusion?
A)The Kelvin thermometer is incorrect.
B)The Celsius thermometer is incorrect.
C)The Fahrenheit thermometer is incorrect.
D)All three thermometers are incorrect.
E)The three thermometers are at different temperatures.
A)The Kelvin thermometer is incorrect.
B)The Celsius thermometer is incorrect.
C)The Fahrenheit thermometer is incorrect.
D)All three thermometers are incorrect.
E)The three thermometers are at different temperatures.
The Fahrenheit thermometer is incorrect.
3
Absolute zero on the Celsius temperature scale is -273.15 °C. What is absolute zero on the Fahrenheit temperature scale?
A)-331.67 °F
B)-363.67 °F
C)-395.67 °F
D)-427.67 °F
E)-459.67 °F
A)-331.67 °F
B)-363.67 °F
C)-395.67 °F
D)-427.67 °F
E)-459.67 °F
-459.67 °F
4
Complete the following statement: Bimetallic strips used as adjustable switches in electric appliances consist of metallic strips that must have different
A)mass.
B)length.
C)volume.
D)expansion coefficients.
E)specific heat capacities.
A)mass.
B)length.
C)volume.
D)expansion coefficients.
E)specific heat capacities.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
5
The coefficient of linear expansion of a certain solid is 11 × 10-6/C°. Assuming this solid behaves like most solids, what is its coefficient of volume expansion?
A)22 × 10-6/C°
B)33 × 10-36/C°
C)33 × 10-6/C°
D)13 × 10-5/C°
E)13 × 10-4/C°
A)22 × 10-6/C°
B)33 × 10-36/C°
C)33 × 10-6/C°
D)13 × 10-5/C°
E)13 × 10-4/C°

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
6
The digital sign outside a local bank reports that the temperature is 41 °C. What is the temperature in degrees Fahrenheit?
A)79 °F
B)99 °F
C)106 °F
D)111 °F
E)120 °F
A)79 °F
B)99 °F
C)106 °F
D)111 °F
E)120 °F

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
7
During an evening news broadcast in Helsinki, Finland, the meteorologist indicated that the day's lowest temperature was -6.0 °C. What is the corresponding value of this temperature on the Fahrenheit scale?
A)-7.2 °F
B)-4.0 °F
C)21 °F
D)25 °F
E)39 °F
A)-7.2 °F
B)-4.0 °F
C)21 °F
D)25 °F
E)39 °F

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
8
Which one of the following statements is the best explanation for the fact that metal pipes that carry water often burst during cold winter months?
A)Water contracts upon freezing while the metal expands at lower temperatures.
B)The metal contracts to a greater extent than the water.
C)The interior of the pipe contracts less than the outside of the pipe.
D)Water expands upon freezing while the metal contracts at lower temperatures.
E)Both the metal and the water expand, but the water expands to a greater extent.
A)Water contracts upon freezing while the metal expands at lower temperatures.
B)The metal contracts to a greater extent than the water.
C)The interior of the pipe contracts less than the outside of the pipe.
D)Water expands upon freezing while the metal contracts at lower temperatures.
E)Both the metal and the water expand, but the water expands to a greater extent.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
9
Zirconium tungstate is an unusual material because its volume shrinks with an increase in temperature for the temperature range 0.3 K to 1050 K (where it decomposes). In fact, the volumetric coefficient of thermal expansion is -26.4 × 10-6/K. Determine the ratio V/V0 for the above mentioned temperature range. Express your answer in percent.
A)-5.28%
B)-3.59%
C)-2.77%
D)-1.90%
E)-1.04%
A)-5.28%
B)-3.59%
C)-2.77%
D)-1.90%
E)-1.04%

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
10
Which one of the following statements explains why it is difficult to measure the coefficient of volume expansion for a liquid?
A)Liquids are more compact than solids.
B)Liquids are more compact than gases.
C)Liquids tend to expand more slowly than solids.
D)The liquid will lose heat to the containing vessel.
E)The volume of the containing vessel will also increase.
A)Liquids are more compact than solids.
B)Liquids are more compact than gases.
C)Liquids tend to expand more slowly than solids.
D)The liquid will lose heat to the containing vessel.
E)The volume of the containing vessel will also increase.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
11
Which one of the following temperatures is approximately equal to the typical temperature of a classroom?
A)0 K
B)0 °C
C)100 °C
D)100 K
E)293 K
A)0 K
B)0 °C
C)100 °C
D)100 K
E)293 K

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
12
Three thermometers are placed in a closed, insulated box and are allowed to reach thermal equilibrium. One is calibrated in Fahrenheit degrees, one in Celsius degrees, and one in Kelvins. The Celsius thermometer reads -40 °C and the Kelvin thermometer reads 233 K. Which one of the following statements is necessarily true?
A)The Kelvin thermometer should read -233 K.
B)The Kelvin thermometer should read -313 K.
C)The Fahrenheit thermometer must read -40 °F.
D)If water were found within the box, it must be in the liquid state.
E)If the temperature of the contents is increased by 10 C°, the reading on the Kelvin thermometer should increase by 273 K.
A)The Kelvin thermometer should read -233 K.
B)The Kelvin thermometer should read -313 K.
C)The Fahrenheit thermometer must read -40 °F.
D)If water were found within the box, it must be in the liquid state.
E)If the temperature of the contents is increased by 10 C°, the reading on the Kelvin thermometer should increase by 273 K.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
13
An aluminum plate has a length of 0.12 m and a width of 0.10 m at 25 °C. The plate is uniformly heated to 225 °C. If the linear expansion coefficient for aluminum is 2.3 × 10-5/C°, what is the change in the area of the plate as a result of the increase in temperature?
A)1.2 × 10-4 m2
B)6.1 × 10-4 m2
C)3.2 × 10-5 m2
D)4.9 × 10-6 m2
E)7.8 × 10-6 m2
A)1.2 × 10-4 m2
B)6.1 × 10-4 m2
C)3.2 × 10-5 m2
D)4.9 × 10-6 m2
E)7.8 × 10-6 m2

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
14
Complete the following statement: A temperature decrease of 30 C° is equal to a temperature decrease of
A)30 F°.
B)30 K.
C)17 F°.
D)26 F°.
E)303 K.
A)30 F°.
B)30 K.
C)17 F°.
D)26 F°.
E)303 K.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
15
A metal rod 40.0000-cm long at 55.0 °C is heated to 85.0 °C. The length of the rod is then measured to be 40.0264 cm. What is the coefficient of linear expansion of the metal?
A)13 × 10-6/C°
B)22 × 10-6/C°
C)44 × 10-6/C°
D)53 × 10-6/C°
E)71 × 10-6/C°
A)13 × 10-6/C°
B)22 × 10-6/C°
C)44 × 10-6/C°
D)53 × 10-6/C°
E)71 × 10-6/C°

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
16
At a certain temperature, a simple pendulum has a period of 1.500 seconds. The support wire is made of brass and has a coefficient of linear thermal expansion of 1.90 × 10-5/C°. How much must the temperature be increased to increase the period to 1.506 seconds?
A)118 C°
B)221 C°
C)316 C°
D)422 C°
E)528 C°
A)118 C°
B)221 C°
C)316 C°
D)422 C°
E)528 C°

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
17
Which one of the following would probably not be used as a temperature sensitive property in the construction of a thermometer?
A)the change in mass of a solid
B)the change in volume of a liquid
C)the change in length of a metal rod
D)the change in electrical resistance of a wire
E)the change in pressure of a gas at constant volume
A)the change in mass of a solid
B)the change in volume of a liquid
C)the change in length of a metal rod
D)the change in electrical resistance of a wire
E)the change in pressure of a gas at constant volume

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
18
A circular hole in an copper plate is 2.925 cm in diameter at 20.0 °C. What is the diameter of the hole if the temperature of the plate is raised to 120.0 °C? The coefficient of linear expansion of copper is 17 × 10-6/C°.
A)2.925 cm
B)2.929 cm
C)2.933 cm
D)2.957 cm
E)2.988 cm
A)2.925 cm
B)2.929 cm
C)2.933 cm
D)2.957 cm
E)2.988 cm

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
19
The coefficient of linear expansion of steel is 12 × 10-6/C°. A railroad track is made of individual rails of steel 1.0 km in length. By what length would these rails change between a cold day when the temperature is -10 °C and a hot day at 30 °C?
A)0.62 cm
B)24 cm
C)48 cm
D)480 cm
E)620 cm
A)0.62 cm
B)24 cm
C)48 cm
D)480 cm
E)620 cm

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
20
A thin, circular disc is made of lead and has a radius of 0.0350 cm at 20.0 °C. Determine the change in the area of the circle if the temperature is increased to 625.0 °C. The coefficient of linear thermal expansion for lead is 29.0 × 10-6/C°.
A)4.33 × 10-5 cm2
B)1.36 × 10-4 cm2
C)1.89 × 10-4 cm2
D)3.19 × 10-4 cm2
E)5.92 × 10-4 cm2
A)4.33 × 10-5 cm2
B)1.36 × 10-4 cm2
C)1.89 × 10-4 cm2
D)3.19 × 10-4 cm2
E)5.92 × 10-4 cm2

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
21
A 2.00-kg metal block slides on a rough, horizontal surface inside an insulated pipe. After sliding a distance of 500.0 m, its temperature is increased by 2.00 °C. Note: Assume that all of the heat generated by frictional heating goes into the metal block. For this metal, the specific heat capacity is 0.150 cal/(g · C°). 
How much work does the force of friction do on the block?
A)zero joules
B)300 J
C)-300 J
D)2510 J
E)-2510 J

How much work does the force of friction do on the block?
A)zero joules
B)300 J
C)-300 J
D)2510 J
E)-2510 J

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
22
Complete the following statement: The term heat most accurately describes
A)the internal energy of an object.
B)a measure of how hot an object is.
C)the absolute temperature of an object.
D)the molecular motion inside of an object.
E)the flow of energy due to a temperature difference.
A)the internal energy of an object.
B)a measure of how hot an object is.
C)the absolute temperature of an object.
D)the molecular motion inside of an object.
E)the flow of energy due to a temperature difference.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
23
Complete the following statement: When a substance undergoes fusion it
A)freezes.
B)sublimes.
C)condenses.
D)vaporizes.
E)evaporates.
A)freezes.
B)sublimes.
C)condenses.
D)vaporizes.
E)evaporates.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
24
Heat is added to a substance, but its temperature does not rise. Which one of the following statements provides the best explanation for this observation?
A)The substance must be a gas.
B)The substance must be a non-perfect solid.
C)The substance undergoes a change of phase.
D)The substance has unusual thermal properties.
E)The substance must be cooler than its environment.
A)The substance must be a gas.
B)The substance must be a non-perfect solid.
C)The substance undergoes a change of phase.
D)The substance has unusual thermal properties.
E)The substance must be cooler than its environment.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
25
A 2.00-kg metal object requires 1.00 × 104 J of heat to raise its temperature from 20.0 °C to 40.0 °C. What is the specific heat capacity of the metal?
A)50.0 J/(kg · C°)
B)125 J/(kg · C°)
C)250 J/(kg · C°)
D)500 J/(kg · C°)
E)1.00 × 103 J/(kg · C°)
A)50.0 J/(kg · C°)
B)125 J/(kg · C°)
C)250 J/(kg · C°)
D)500 J/(kg · C°)
E)1.00 × 103 J/(kg · C°)

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
26
A gold sphere has a radius of 1.000 cm at 25.0 °C. If 7650 J of heat is added to the sphere, what will the final volume of the sphere be? Gold has a density of 19 300 kg/m3 at 25.0 °C, a specific heat capacity of 129 J/(kg · C°), and a coefficient of volume expansion of 42.0 × 10-6/C°.
A)2.88 × 10-6 m3
B)3.01 × 10-6 m3
C)3.33 × 10-6 m3
D)3.91 × 10-6 m3
E)4.32 × 10-6 m3
A)2.88 × 10-6 m3
B)3.01 × 10-6 m3
C)3.33 × 10-6 m3
D)3.91 × 10-6 m3
E)4.32 × 10-6 m3

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
27
A 200.0-kg object is attached via an ideal pulley system to paddle wheels that are submerged in 0.480 kg of glycerin at 20.0 °C in an insulated container as shown. Then, the object falls through a distance of 5.00 m causing the paddle wheel to turn. Assuming all of the mechanical energy lost by the falling object goes into the water, determine the final temperature of the glycerin. The specific heat capacity of glycerin is 2410 J/ 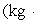 Co).
Co). 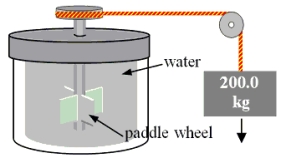
A)4.90 °C
B)28.5 °C
C)24.9 °C
D)40.4 °C
E)8.47 °C
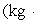 Co).
Co). 
A)4.90 °C
B)28.5 °C
C)24.9 °C
D)40.4 °C
E)8.47 °C

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
28
Which one of the following phrases is an example of sublimation?
A)the fumes emitted from moth balls
B)the mist produced by liquid nitrogen
C)the formation of dew on blades of grass
D)the formation of raindrops in the atmosphere
E)the condensation of steam on a kitchen window
A)the fumes emitted from moth balls
B)the mist produced by liquid nitrogen
C)the formation of dew on blades of grass
D)the formation of raindrops in the atmosphere
E)the condensation of steam on a kitchen window

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
29
The coefficient of volumetric expansion for gold is 4.20 × 10-5/C°. The density of gold is 19 300 kg/m3 at 0.0 °C. What is the density of gold at 1050 °C?
A)20 200 kg/ m3
B)19 300 kg/m3
C)19 000 kg/m3
D)18 800 kg/m3
E)18 500 kg/m3
A)20 200 kg/ m3
B)19 300 kg/m3
C)19 000 kg/m3
D)18 800 kg/m3
E)18 500 kg/m3

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
30
Two cubes, one silver and one iron, have the same mass and temperature. A quantity Q of heat is removed from each cube. Which one of the following properties causes the final temperatures of the cubes to be different?
A)density
B)latent heat of vaporization
C)specific heat capacity
D)coefficient of volume expansion
E)volume
A)density
B)latent heat of vaporization
C)specific heat capacity
D)coefficient of volume expansion
E)volume

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
31
A 2.0-g sample of steam at 100 °C loses 1140 calories of heat. What is the resulting temperature of the sample?
A)60 °C
B)70 °C
C)80 °C
D)96 °C
E)99 °C
A)60 °C
B)70 °C
C)80 °C
D)96 °C
E)99 °C

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
32
Complete the following statement: When solid NH3 passes directly to the gaseous state it is said to
A)melt.
B)sublime.
C)condense.
D)evaporate.
E)fuse.
A)melt.
B)sublime.
C)condense.
D)evaporate.
E)fuse.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
33
Two spheres, labeled A and B, have identical masses, but are made of different substances. The specific heat capacity of sphere A is 645 J/(kg · C°) and that of sphere B is 240 J/(kg · C°). The spheres are initially at 21 °C; and the same quantity of heat is added to each sphere. If the final temperature of sphere A is 74 °C, what is the approximate final temperature of sphere B?
A)160 °C
B)140 °C
C)110 °C
D)53 °C
E)39 °C
A)160 °C
B)140 °C
C)110 °C
D)53 °C
E)39 °C

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
34
A 2.00-kg metal block slides on a rough, horizontal surface inside an insulated pipe. After sliding a distance of 500.0 m, its temperature is increased by 2.00 °C. Note: Assume that all of the heat generated by frictional heating goes into the metal block. For this metal, the specific heat capacity is 0.150 cal/(g · C°). 
What is the coefficient of sliding friction between the block and the surface?
A)zero
B)0.061
C)0.100
D)0.256
E)0.299

What is the coefficient of sliding friction between the block and the surface?
A)zero
B)0.061
C)0.100
D)0.256
E)0.299

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
35
A soft drink manufacturer claims that a new diet drink is "low Joule." The label indicates the available energy per serving is 6300 J. What is the equivalent of this energy in Calories (1 Calorie = 1000 cal)?
A)0.015 Cal
B)0.48 Cal
C)1.0 Cal
D)1.5 Cal
E)4.8 Cal
A)0.015 Cal
B)0.48 Cal
C)1.0 Cal
D)1.5 Cal
E)4.8 Cal

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
36
A tanker ship is filled with 2.25 × 105 m3 of gasoline at a refinery in southern Texas when the temperature is 17.2 °C. When the ship arrives in New York City, the temperature is 1.3 °C. If the coefficient of volumetric expansion for gasoline is 9.50 × 10-4/C°, how much has the volume of the gasoline decreased when it is unloaded in New York?
A)1.50 × 10-2 m3
B)66.2 m3
C)1290 m3
D)3400 m3
E)1.05 × 104 m3
A)1.50 × 10-2 m3
B)66.2 m3
C)1290 m3
D)3400 m3
E)1.05 × 104 m3

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
37
The specific heat capacity of iron is approximately half that of aluminum. Two balls of equal mass, one made of iron and the other of aluminum, both at 90 °C, are dropped into a thermally insulated jar that contains an equal mass of water at 25 °C. Thermal equilibrium is eventually reached. Which one of the following statements concerning the final temperatures is true?
A)Both balls will reach the same final temperature.
B)The iron ball will reach a higher final temperature than the aluminum ball.
C)The aluminum ball will reach a higher final temperature than the iron ball.
D)The difference in the final temperatures of the balls depends on the initial mass of the water.
E)The difference in the final temperatures of the balls depends on the initial temperature of the water.
A)Both balls will reach the same final temperature.
B)The iron ball will reach a higher final temperature than the aluminum ball.
C)The aluminum ball will reach a higher final temperature than the iron ball.
D)The difference in the final temperatures of the balls depends on the initial mass of the water.
E)The difference in the final temperatures of the balls depends on the initial temperature of the water.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
38
A 0.30-kg lead ball is heated to 90.0 °C and dropped into an ideal calorimeter containing 0.50 kg of water initially at 20.0 °C. What is the final equilibrium temperature of the lead ball? The specific heat capacity of lead is 128 J/(kg · C°); and the specific heat of water is 4186 J/(kg · C°).
A)84.8 °C
B)20.8 °C
C)21.3 °C
D)27.8 °C
E)32.1 °C
A)84.8 °C
B)20.8 °C
C)21.3 °C
D)27.8 °C
E)32.1 °C

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
39
An aluminum tank of volume 0.0300 m3 is filled to the top with mercury at 20.0 °C. The tank is placed inside a chamber with an interior temperature of 70.0 °C. The coefficient of volume expansion for mercury is 1.82 × 10-4/C°; and the coefficient of linear expansion of aluminum is 23.0 × 10-6/C°. After the tank and its contents reach thermal equilibrium with the interior of the chamber, how much mercury has spilled?
A)2.52 × 10-5 m3
B)6.60 × 10-5 m3
C)1.69 × 10-4 m3
D)1.92 × 10-4 m3
E)2.00 × 10-4 m3
A)2.52 × 10-5 m3
B)6.60 × 10-5 m3
C)1.69 × 10-4 m3
D)1.92 × 10-4 m3
E)2.00 × 10-4 m3

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
40
The units of heat are equivalent to those of which one of the following quantities?
A)force/time
B)work
C)temperature
D)specific heat capacity
E)power
A)force/time
B)work
C)temperature
D)specific heat capacity
E)power

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
41
A 4.0-g sample of ice at 0.0 °C falls through a distance of 30.0 m and undergoes a completely inelastic collision with the earth. If all of the mechanical energy is absorbed by the ice, how much of it melts?
A)2.9 × 10-3 g
B)3.3 × 10-3 g
C)7.6 × 10-3 g
D)1.8 × 10-2 g
E)2.1 × 10-2 g
A)2.9 × 10-3 g
B)3.3 × 10-3 g
C)7.6 × 10-3 g
D)1.8 × 10-2 g
E)2.1 × 10-2 g

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
42
A 0.0500-kg lead bullet of volume 5.00 × 10–6 m3 at 20.0 °C hits a block that is made of an ideal thermal insulator and comes to rest at its center. At that time, the temperature of the bullet is 327 °C. Use the following information for lead: 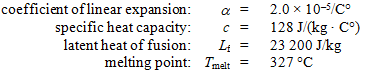
What is the volume of the bullet when it comes to rest?
A)5.00 × 10-6 m3
B)5.01 × 10-6 m3
C)5.03 × 10-6 m3
D)5.07 × 10-6 m3
E)5.09 × 10-6 m3
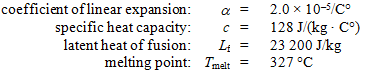
What is the volume of the bullet when it comes to rest?
A)5.00 × 10-6 m3
B)5.01 × 10-6 m3
C)5.03 × 10-6 m3
D)5.07 × 10-6 m3
E)5.09 × 10-6 m3

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
43
A household humidifier continuously takes water in at 20.0 °C at a rate of 5.60 × 10-5 kg/s and heats it until it evaporates. If the cost of electricity is $ 0.14/kWh, what is the daily cost of operating the humidifier? Notes: one kWh = 3.60 × 106 J; and for water, the specific heat capacity is 4186 J/(kg · C°); the latent heats of fusion and vaporization are 3.35 × 105 J/kg and 2.26 × 106 J/kg, respectively.
A)$ 0.31
B)$ 0.49
C)$ 0.58
D)$ 0.65
E)$ 0.70
A)$ 0.31
B)$ 0.49
C)$ 0.58
D)$ 0.65
E)$ 0.70

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
44
What is the minimum amount of energy required to completely melt a 7.25-kg lead brick which has a starting temperature of 18.0 °C? The melting point of lead is 328 °C. The specific heat capacity of lead is 128 J/(kg · C°); and its latent heat of fusion is 23 200 J/kg.
A)1.20 × 105 J
B)1.68 × 105 J
C)2.88 × 105 J
D)4.56 × 105 J
E)7.44 × 105 J
A)1.20 × 105 J
B)1.68 × 105 J
C)2.88 × 105 J
D)4.56 × 105 J
E)7.44 × 105 J

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
45
An ordinary mercury thermometer at room temperature is quickly placed in a beaker of hot water. The mercury column is observed to drop slightly before it rises to the final equilibrium temperature. Which one of the following statements is the best explanation for this behavior?
A)The glass envelope expands before the heat reaches the mercury.
B)The expansion coefficient of glass is larger than that of mercury.
C)Both the mercury and the glass initially expand, but at different rates.
D)Initially, the mercury contracts.
E)Initially, the glass envelop contracts.
A)The glass envelope expands before the heat reaches the mercury.
B)The expansion coefficient of glass is larger than that of mercury.
C)Both the mercury and the glass initially expand, but at different rates.
D)Initially, the mercury contracts.
E)Initially, the glass envelop contracts.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
46
A liquid is in equilibrium with its vapor in a closed vessel. Which one of the following statements is necessarily true?
A)The rate of condensation is greater than the evaporation rate.
B)The rate of evaporation is greater than the condensation rate.
C)The temperature of the vapor is greater than that of the liquid.
D)Molecules of the liquid do not have enough energy to vaporize.
E)The temperature of the vapor is the same as that of the liquid.
A)The rate of condensation is greater than the evaporation rate.
B)The rate of evaporation is greater than the condensation rate.
C)The temperature of the vapor is greater than that of the liquid.
D)Molecules of the liquid do not have enough energy to vaporize.
E)The temperature of the vapor is the same as that of the liquid.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
47
A 0.0500-kg lead bullet of volume 5.00 × 10–6 m3 at 20.0 °C hits a block that is made of an ideal thermal insulator and comes to rest at its center. At that time, the temperature of the bullet is 327 °C. Use the following information for lead: 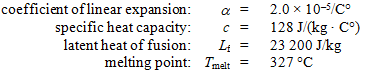
What additional heat would be needed to melt the bullet?
A)420 J
B)628 J
C)837 J
D)1160 J
E)2010 J
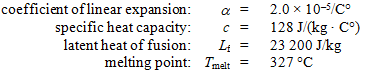
What additional heat would be needed to melt the bullet?
A)420 J
B)628 J
C)837 J
D)1160 J
E)2010 J

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
48
The graph shows the equilibrium vapor pressure versus temperature for a certain liquid and its vapor within an open container. If the container is at sea level, at approximately what temperature will the liquid boil? 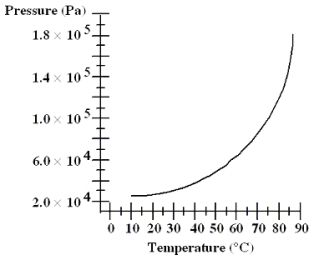
A)50 °C
B)65 °C
C)75 °C
D)85 °C
E)100 °C

A)50 °C
B)65 °C
C)75 °C
D)85 °C
E)100 °C

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
49
On a warm summer day, the relative humidity is 30 % when the temperature is 32 °C. Which one of the following statements is true if the temperature suddenly decreases to 26 °C and all other conditions remain the same?
A)The relative humidity will decrease.
B)The relative humidity will increase.
C)The dew point will change.
D)The partial pressure of water vapor will decrease.
E)The vaporization curve of water will change.
A)The relative humidity will decrease.
B)The relative humidity will increase.
C)The dew point will change.
D)The partial pressure of water vapor will decrease.
E)The vaporization curve of water will change.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
50
A 0.040-kg ice cube at 0 °C is placed in an insulated box that contains 0.0075 kg of steam at 100 °C. What is the equilibrium temperature reached by this closed system? Note: Assume that all of the ice melts. 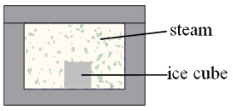
A)22.7 °C
B)33.6 °C
C)44.9 °C
D)50.7 °C
E)66.4 °C

A)22.7 °C
B)33.6 °C
C)44.9 °C
D)50.7 °C
E)66.4 °C

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
51
Determine the latent heat of vaporization of unknown substance X in kcal/g if 4.0 g of boiling liquid X are completely vaporized in 1.2 hours by an input of 15 kcal/h into the system by an energy source.
A)4.5 kcal/g
B)1.5 kcal/g
C)2.7 kcal/g
D)3.0 kcal/g
E)5.9 kcal/g
A)4.5 kcal/g
B)1.5 kcal/g
C)2.7 kcal/g
D)3.0 kcal/g
E)5.9 kcal/g

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
52
Using the data in the table, determine how many calories are needed to change 100 g of solid X at 10 °C to a vapor at 210 °C. 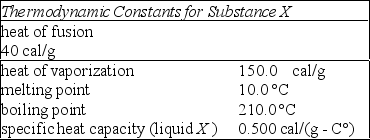
A)4000 cal
B)10 000 cal
C)15 000 cal
D)29 000 cal
E)39 000 cal

A)4000 cal
B)10 000 cal
C)15 000 cal
D)29 000 cal
E)39 000 cal

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
53
A thermos bottle contains 3.0 kg of water and 2.0 kg of ice in thermal equilibrium at 0 °C. How much heat is required to bring the system to thermal equilibrium at 50 °C?
A)1.05 × 106 J
B)1.30 × 106 J
C)1.72 × 106 J
D)2.26 × 106 J
E)1.13 × 107 J
A)1.05 × 106 J
B)1.30 × 106 J
C)1.72 × 106 J
D)2.26 × 106 J
E)1.13 × 107 J

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
54
Heat is added to a sample of water in an insulated container. Which one of the following statements is necessarily true?
A)The temperature of the water will rise.
B)The volume of the water must decrease.
C)The mass of the system must decrease.
D)Under certain conditions, the temperature of the water can decrease.
E)The type of change that will occur depends on the original temperature of the water.
A)The temperature of the water will rise.
B)The volume of the water must decrease.
C)The mass of the system must decrease.
D)Under certain conditions, the temperature of the water can decrease.
E)The type of change that will occur depends on the original temperature of the water.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
55
A 0.0500-kg lead bullet of volume 5.00 × 10–6 m3 at 20.0 °C hits a block that is made of an ideal thermal insulator and comes to rest at its center. At that time, the temperature of the bullet is 327 °C. Use the following information for lead: 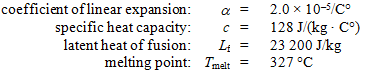
How much heat was needed to raise the bullet to its final temperature?
A)963 J
B)1960 J
C)3640 J
D)3880 J
E)4440 J
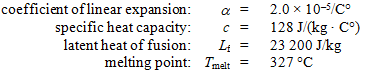
How much heat was needed to raise the bullet to its final temperature?
A)963 J
B)1960 J
C)3640 J
D)3880 J
E)4440 J

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
56
In an insulated container, 0.50 kg of steam, initially at 140 °C, is mixed with 2.0 kg of ice, initially at -20.0 °C. What is the final temperature inside the container if heat exchanges with the container are ignored?
A)16 °C
B)50 °C
C)60 °C
D)64 °C
E)86 °C
A)16 °C
B)50 °C
C)60 °C
D)64 °C
E)86 °C

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
57
Given the following information, determine the relative humidity at 15 °C. 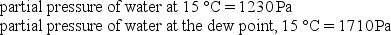
A)39.0 %
B)47.9 %
C)50.8 %
D)64.1 %
E)71.9 %
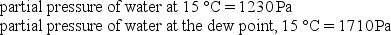
A)39.0 %
B)47.9 %
C)50.8 %
D)64.1 %
E)71.9 %

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
58
Ryan places 0.150 kg of boiling water in a thermos bottle. How many kilograms of ice at -12.0 °C must Ryan add to the thermos so that the equilibrium temperature of the water is 75.0 °C?
A)0.0233 kg
B)0.0265 kg
C)0.0436 kg
D)0.0713 kg
E)0.625 kg
A)0.0233 kg
B)0.0265 kg
C)0.0436 kg
D)0.0713 kg
E)0.625 kg

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
59
Which would cause a more serious burn: 30 g of steam or 30 g of liquid water, both at 100 °C; and why is this so?
A)Water, because it is denser than steam.
B)Steam, because of its specific heat capacity.
C)Steam, because of its latent heat of vaporization.
D)Water, because its specific heat is greater than that of steam.
E)Either one would cause a burn of the same severity since they are both at the same temperature.
A)Water, because it is denser than steam.
B)Steam, because of its specific heat capacity.
C)Steam, because of its latent heat of vaporization.
D)Water, because its specific heat is greater than that of steam.
E)Either one would cause a burn of the same severity since they are both at the same temperature.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
60
A 0.030-kg ice cube at 0 °C is placed in an insulated box that contains a fixed quantity of steam at 100 °C. When thermal equilibrium of this closed system is established, its temperature is found to be 23 °C. Determine the original mass of the steam at 100 °C. 
A)0.17 g
B)1.7 g
C)2.5 g
D)4.8 g
E)5.0 g

A)0.17 g
B)1.7 g
C)2.5 g
D)4.8 g
E)5.0 g

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
61
After working a 0.55-kg iron horseshoe with a temperature of 460 °C on an anvil, a ferrier drops it into a bucket that contains 11 kg of water with an initial temperature of 24 °C. Assuming no heat is transferred to the environment, determine the final temperature of the horseshoe in the bucket of water when thermal equilibrium is achieved. The specific heat capacity of iron is 452 J/kg · C°. For water, the specific heat capacity is 4186 J/kg · C°.
A)26 °C
B)34 °C
C)41 °C
D)49 °C
E)52 °C
A)26 °C
B)34 °C
C)41 °C
D)49 °C
E)52 °C

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
62
Heat is added to a 1.0-kg solid sample of a material at -200 °C. The figure shows the temperature of the material as a function of the heat added. 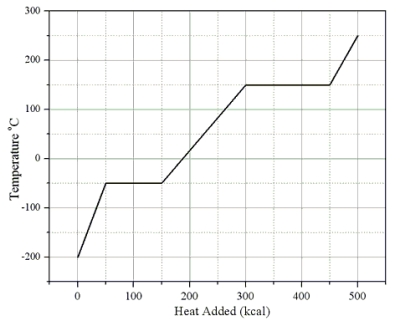
What is the latent heat of fusion of this material?
A)50 cal/g
B)100 cal/g
C)150 cal/g
D)300 cal/g
E)450 cal/g

What is the latent heat of fusion of this material?
A)50 cal/g
B)100 cal/g
C)150 cal/g
D)300 cal/g
E)450 cal/g

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
63
Heat is added to a 1.0-kg solid sample of a material at -200 °C. The figure shows the temperature of the material as a function of the heat added. 
What is the specific heat capacity of this substance in its liquid state?
A)0.33 cal/(g · C°)
B)0.75 cal/(g · C°)
C)1.00 cal/(g · C°)
D)1.33 cal/(g · C°)
E)3.00 cal/(g · C°)

What is the specific heat capacity of this substance in its liquid state?
A)0.33 cal/(g · C°)
B)0.75 cal/(g · C°)
C)1.00 cal/(g · C°)
D)1.33 cal/(g · C°)
E)3.00 cal/(g · C°)

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
64
Heat is added to a 1.0-kg solid sample of a material at -200 °C. The figure shows the temperature of the material as a function of the heat added. 
What is the latent heat of vaporization of this material?
A)50 cal/g
B)100 cal/g
C)150 cal/g
D)300 cal/g
E)450 cal/g

What is the latent heat of vaporization of this material?
A)50 cal/g
B)100 cal/g
C)150 cal/g
D)300 cal/g
E)450 cal/g

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
65
Heat is added to a 1.0-kg solid sample of a material at -200 °C. The figure shows the temperature of the material as a function of the heat added. 
What is the specific heat capacity of this substance in its solid state?
A)0.33 cal/(g · C°)
B)0.75 cal/(g · C°)
C)1.00 cal/(g · C°)
D)1.33 cal/(g · C°)
E)3.00 cal/(g · C°)

What is the specific heat capacity of this substance in its solid state?
A)0.33 cal/(g · C°)
B)0.75 cal/(g · C°)
C)1.00 cal/(g · C°)
D)1.33 cal/(g · C°)
E)3.00 cal/(g · C°)

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
66
Heat is added to a 1.0-kg solid sample of a material at -200 °C. The figure shows the temperature of the material as a function of the heat added. 
Which one of the following statements concerning this substance is true?
A)It boils at 300 °C.
B)It melts at -200 °C.
C)It is a liquid at 200 °C.
D)It can coexist as a solid and a liquid at -50 °C.
E)It can exist as a solid, liquid, and gas at 150 °C.

Which one of the following statements concerning this substance is true?
A)It boils at 300 °C.
B)It melts at -200 °C.
C)It is a liquid at 200 °C.
D)It can coexist as a solid and a liquid at -50 °C.
E)It can exist as a solid, liquid, and gas at 150 °C.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck


