Exam 12: Temperature and Heat
Exam 1: Introduction and Mathematical Concepts70 Questions
Exam 2: Kinematics in One Dimension103 Questions
Exam 3: Kinematics in Two Dimensions68 Questions
Exam 4: Forces and Newtons Laws of Motion103 Questions
Exam 5: Dynamics of Uniform Circular Motion59 Questions
Exam 6: Work and Energy78 Questions
Exam 7: Impulse and Momentum66 Questions
Exam 8: Rotational Kinematics55 Questions
Exam 9: Rotational Dynamics57 Questions
Exam 10: Simple Harmonic Motion and Elasticity63 Questions
Exam 11: Fluids65 Questions
Exam 12: Temperature and Heat66 Questions
Exam 13: The Transfer of Heat42 Questions
Exam 14: The Ideal Gas Law and Kinetic Theory55 Questions
Exam 15: Thermodynamics79 Questions
Exam 16: Waves and Sound67 Questions
Exam 17: The Principle of Linear Superposition and Interference Phenomena46 Questions
Exam 18: Electric Forces and Electric Fields61 Questions
Exam 19: Electric Potential Energy and the Electric Potential70 Questions
Exam 20: Electric Circuits100 Questions
Exam 21: Magnetic Forces and Magnetic Fields66 Questions
Exam 22: Electromagnetic Induction71 Questions
Exam 23: Alternating Current Circuits84 Questions
Exam 24: Electromagnetic Waves66 Questions
Exam 25: The Refl Ection of Light: Mirrors43 Questions
Exam 26: The Refraction of Light: Lenses and Optical Instruments102 Questions
Exam 27: Interference and the Wave Nature of Light57 Questions
Exam 28: Special Relativity63 Questions
Exam 29: Particles and Waves54 Questions
Exam 30: The Nature of the Atom74 Questions
Exam 31: Nuclear Physics and Radioactivity37 Questions
Exam 32: Ionizing Radiation, Nuclear Energy, and Elementary Particles45 Questions
Select questions type
A household humidifier continuously takes water in at 20.0 °C at a rate of 5.60 × 10-5 kg/s and heats it until it evaporates. If the cost of electricity is $ 0.14/kWh, what is the daily cost of operating the humidifier? Notes: one kWh = 3.60 × 106 J; and for water, the specific heat capacity is 4186 J/(kg · C°); the latent heats of fusion and vaporization are 3.35 × 105 J/kg and 2.26 × 106 J/kg, respectively.
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
B
Complete the following statement: The term heat most accurately describes
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
E
Which one of the following statements is the best explanation for the fact that metal pipes that carry water often burst during cold winter months?
Free
(Multiple Choice)
5.0/5  (41)
(41)
Correct Answer:
D
During an evening news broadcast in Helsinki, Finland, the meteorologist indicated that the day's lowest temperature was -6.0 °C. What is the corresponding value of this temperature on the Fahrenheit scale?
(Multiple Choice)
4.8/5  (41)
(41)
An ordinary mercury thermometer at room temperature is quickly placed in a beaker of hot water. The mercury column is observed to drop slightly before it rises to the final equilibrium temperature. Which one of the following statements is the best explanation for this behavior?
(Multiple Choice)
4.7/5  (38)
(38)
Which one of the following phrases is an example of sublimation?
(Multiple Choice)
4.9/5  (34)
(34)
Two cubes, one silver and one iron, have the same mass and temperature. A quantity Q of heat is removed from each cube. Which one of the following properties causes the final temperatures of the cubes to be different?
(Multiple Choice)
4.7/5  (38)
(38)
The coefficient of linear expansion of a certain solid is 11 × 10-6/C°. Assuming this solid behaves like most solids, what is its coefficient of volume expansion?
(Multiple Choice)
4.9/5  (37)
(37)
Heat is added to a 1.0-kg solid sample of a material at -200 °C. The figure shows the temperature of the material as a function of the heat added. 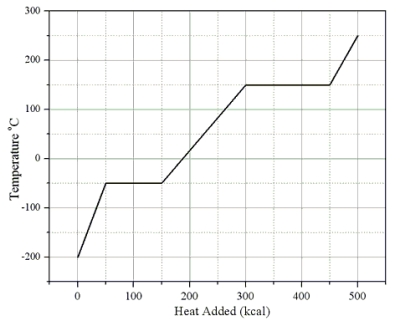 -What is the latent heat of vaporization of this material?
-What is the latent heat of vaporization of this material?
(Multiple Choice)
4.8/5  (33)
(33)
Complete the following statement: Bimetallic strips used as adjustable switches in electric appliances consist of metallic strips that must have different
(Multiple Choice)
5.0/5  (34)
(34)
After working a 0.55-kg iron horseshoe with a temperature of 460 °C on an anvil, a ferrier drops it into a bucket that contains 11 kg of water with an initial temperature of 24 °C. Assuming no heat is transferred to the environment, determine the final temperature of the horseshoe in the bucket of water when thermal equilibrium is achieved. The specific heat capacity of iron is 452 J/kg · C°. For water, the specific heat capacity is 4186 J/kg · C°.
(Multiple Choice)
4.9/5  (33)
(33)
Heat is added to a sample of water in an insulated container. Which one of the following statements is necessarily true?
(Multiple Choice)
4.9/5  (36)
(36)
A 4.0-g sample of ice at 0.0 °C falls through a distance of 30.0 m and undergoes a completely inelastic collision with the earth. If all of the mechanical energy is absorbed by the ice, how much of it melts?
(Multiple Choice)
4.8/5  (36)
(36)
A 0.030-kg ice cube at 0 °C is placed in an insulated box that contains a fixed quantity of steam at 100 °C. When thermal equilibrium of this closed system is established, its temperature is found to be 23 °C. Determine the original mass of the steam at 100 °C. 
(Multiple Choice)
4.7/5  (26)
(26)
A steel string guitar is strung so that there is negligible tension in the strings at a temperature of 24.9 °C. The guitar is taken to an outdoor winter concert where the temperature of the strings decreases to -15.1 °C. The cross-sectional area of a particular string is 5.5 × 10-6 m2. The distance between the points where the string is attached does not change. For steel, Young's modulus is 2.0 × 1011 N/m2; and the coefficient of linear expansion is 1.2 × 10-5/C°. Use your knowledge of linear thermal expansion and stress to calculate the tension in the string at the concert.
(Multiple Choice)
5.0/5  (30)
(30)
Which one of the following would probably not be used as a temperature sensitive property in the construction of a thermometer?
(Multiple Choice)
4.8/5  (32)
(32)
Heat is added to a 1.0-kg solid sample of a material at -200 °C. The figure shows the temperature of the material as a function of the heat added.  -What is the specific heat capacity of this substance in its solid state?
-What is the specific heat capacity of this substance in its solid state?
(Multiple Choice)
4.9/5  (33)
(33)
Three thermometers are placed in a closed, insulated box and are allowed to reach thermal equilibrium. One is calibrated in Fahrenheit degrees, one in Celsius degrees, and one in Kelvins. The Celsius thermometer reads -40 °C and the Kelvin thermometer reads 233 K. Which one of the following statements is necessarily true?
(Multiple Choice)
4.8/5  (28)
(28)
Which would cause a more serious burn: 30 g of steam or 30 g of liquid water, both at 100 °C; and why is this so?
(Multiple Choice)
4.7/5  (27)
(27)
The specific heat capacity of iron is approximately half that of aluminum. Two balls of equal mass, one made of iron and the other of aluminum, both at 90 °C, are dropped into a thermally insulated jar that contains an equal mass of water at 25 °C. Thermal equilibrium is eventually reached. Which one of the following statements concerning the final temperatures is true?
(Multiple Choice)
4.9/5  (43)
(43)
Showing 1 - 20 of 66
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)