Deck 31: Nuclear Physics and Radioactivity
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/37
Play
Full screen (f)
Deck 31: Nuclear Physics and Radioactivity
1
What is the wavelength of the 0.059-MeV ray photon emitted by 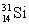 ?
?
A)6.7 × 10-12 m
B)1.4 × 10-11 m
C)2.1 × 10-11 m
D)2.8 × 10-11 m
E)3.4 × 10-11 m
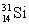 ?
?A)6.7 × 10-12 m
B)1.4 × 10-11 m
C)2.1 × 10-11 m
D)2.8 × 10-11 m
E)3.4 × 10-11 m
2.1 × 10-11 m
2
In a beta decay process, not all of the released energy is carried by the beta particle. Who proposed the existence of the neutrino in 1930 to account for the missing energy?
A)Niels Bohr
B)Erwin Schrödinger
C)Werner Heisenberg
D)Wolfgang Pauli
E)Enrico Fermi
A)Niels Bohr
B)Erwin Schrödinger
C)Werner Heisenberg
D)Wolfgang Pauli
E)Enrico Fermi
Wolfgang Pauli
3
The nucleus of an atom has a radius of 5.61 × 10-15 m. If the nucleus contains 45 protons, how many neutrons does the nucleus contain?
A)45
B)57
C)69
D)84
E)102
A)45
B)57
C)69
D)84
E)102
57
4
This question refers to the figure shown. Which one of the following concepts explains why heavy nuclei do not follow the N = Z line (or trend) in the figure? 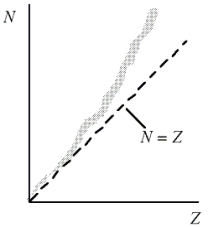
A)transmutation
B)Coulomb repulsion
C)particle-wave duality
D)Pauli exclusion principle
E)Heisenberg uncertainty principle

A)transmutation
B)Coulomb repulsion
C)particle-wave duality
D)Pauli exclusion principle
E)Heisenberg uncertainty principle

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is not an assumption involved in the expression: r = (1.2 × 10-15 m) A1/3?
A)Nuclei are incompressible.
B)The nucleus is spherical in shape.
C)All nucleons have roughly the same mass.
D)Nuclear densities are proportional to the mass numbers.
E)The number of nucleons is proportional to the nuclear mass.
A)Nuclei are incompressible.
B)The nucleus is spherical in shape.
C)All nucleons have roughly the same mass.
D)Nuclear densities are proportional to the mass numbers.
E)The number of nucleons is proportional to the nuclear mass.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
6
Which one of the following descriptive terms does not apply to nuclear forces?
A)strong
B)charge-independent
C)weak
D)short-range
E)long-range
A)strong
B)charge-independent
C)weak
D)short-range
E)long-range

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
7
Which one of the following statements concerning stable nuclei is true?
A)Stable nuclei have nucleon numbers less than 83.
B)Stable nuclei generally have odd atomic numbers.
C)Stable nuclei have atomic numbers greater than 83.
D)Stable nuclei generally have an odd number of neutrons.
E)Stable nuclei generally have more neutrons than protons.
A)Stable nuclei have nucleon numbers less than 83.
B)Stable nuclei generally have odd atomic numbers.
C)Stable nuclei have atomic numbers greater than 83.
D)Stable nuclei generally have an odd number of neutrons.
E)Stable nuclei generally have more neutrons than protons.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
8
Which process is used in the operation of the smoke detector discussed in Cutnell and Johnson's text?
A)alpha decay
B)beta decay
C)gamma decay
D)X-ray absorption
E)proton absorption
A)alpha decay
B)beta decay
C)gamma decay
D)X-ray absorption
E)proton absorption

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
9
Complete the following sentence: In a + decay process, the emitted particle is
A)an electron.
B)a neutron.
C)a positron.
D)a proton.
E)a photon.
A)an electron.
B)a neutron.
C)a positron.
D)a proton.
E)a photon.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
10
Assuming the radius of a hydrogen atom is given by the Bohr radius, rBohr = 5.29 × 10-11 m, what is the ratio of the nuclear density of a hydrogen atom to its atomic density? Note: Assume for this calculation that the mass of the atom is equal to the mass of the proton.
A)1.2 × 10-14
B)4.4 × 104
C)8.6 × 1013
D)3.9 × 1017
E)2.3 × 10-5
A)1.2 × 10-14
B)4.4 × 104
C)8.6 × 1013
D)3.9 × 1017
E)2.3 × 10-5

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
11
Which one of the following quantities is not a conserved quantity according to the laws of physics?
A)electric charge
B)nucleon number
C)angular momentum
D)linear momentum
E)kinetic energy
A)electric charge
B)nucleon number
C)angular momentum
D)linear momentum
E)kinetic energy

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
12
Which one of the following thicknesses of lead would be least effective in stopping rays?
A)0.04 mm
B)0.15 mm
C)0.30 mm
D)0.45 mm
E)0.50 mm
A)0.04 mm
B)0.15 mm
C)0.30 mm
D)0.45 mm
E)0.50 mm

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
13
The binding energy of an isotope of chlorine is 298 MeV. What is the mass defect of this chlorine nucleus in atomic mass units?
A)3.13 u
B)2.30 u
C)0.882 u
D)0.320 u
E)0.034 u
A)3.13 u
B)2.30 u
C)0.882 u
D)0.320 u
E)0.034 u

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
14
What is the approximate radius of a nitrogen nucleus that has seven protons and seven neutrons?
A)1.2 × 10-15 m
B)2.2 × 10-15 m
C)2.7 × 10-15 m
D)2.9 × 10-15 m
E)3.2 × 10-15 m
A)1.2 × 10-15 m
B)2.2 × 10-15 m
C)2.7 × 10-15 m
D)2.9 × 10-15 m
E)3.2 × 10-15 m

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
15
How many neutrons are there in the nucleus 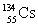 ?
?
A)79
B)55
C)134
D)24
E)120
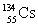 ?
?A)79
B)55
C)134
D)24
E)120

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
16
Which one of the following types of nuclear radiation is not affected by a magnetic field?
A)alpha particles
B) - rays
C)gamma rays
D) + rays
E)helium nuclei
A)alpha particles
B) - rays
C)gamma rays
D) + rays
E)helium nuclei

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
17
Osmium has atomic number 76. A particular isotope of osmium has an atomic mass of 191.961 u. Which symbol correctly represents this isotope?
A)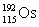
B)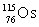
C)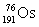
D)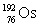
E)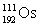
A)
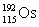
B)
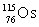
C)
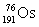
D)
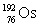
E)
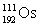

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
18
Radiation or(and) particles emerge(s) from a radioactive sample. These products from the sample are allowed to pass through a narrow slit and may be considered a beam. The beam is passed between two plates that carry opposite electrical charge. The experimental region contains no magnetic fields. It is observed that the beam is deflected toward the negatively charged plate. 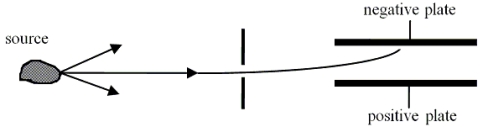 Which one of the following statements is the best conclusion for this situation?
Which one of the following statements is the best conclusion for this situation?
A)The beam is only rays.
B)The beam is only - rays.
C)The beam is only rays.
D)The beam could be either rays or + rays.
E)The beam could be rays, + rays, or rays.
 Which one of the following statements is the best conclusion for this situation?
Which one of the following statements is the best conclusion for this situation?A)The beam is only rays.
B)The beam is only - rays.
C)The beam is only rays.
D)The beam could be either rays or + rays.
E)The beam could be rays, + rays, or rays.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
19
Which entry in the table below describes the daughter nucleus when 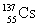 decays by - emission?
decays by - emission? 
A)
B)
C)
D)
E)
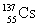 decays by - emission?
decays by - emission? 
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
20
What is the SI unit for activity?
A)Ci
B)counts/min
C)Hz
D)Gy
E)Bq
A)Ci
B)counts/min
C)Hz
D)Gy
E)Bq

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
21
Which process is involved in determining the age of a prehistoric object?
A)alpha decay
B)beta decay
C)gamma decay
D)X-ray absorption
E)proton absorption
A)alpha decay
B)beta decay
C)gamma decay
D)X-ray absorption
E)proton absorption

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
22
The activity of carbon-14 in a sample of charcoal from an archaeological site is 0.04 Bq. Determine the age of the sample. The half-life of carbon-14 is 5730 years.
A)10 500 yr
B)12 500 yr
C)14 500 yr
D)16 500 yr
E)18 500 yr
A)10 500 yr
B)12 500 yr
C)14 500 yr
D)16 500 yr
E)18 500 yr

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
23
Determine the activity of 6.0 × 1012 atoms of Rn-220 that has a half-life of 56 s.
A)2.0 Ci
B)2.5 Ci
C)3.0 Ci
D)3.5 Ci
E)4.0 Ci
A)2.0 Ci
B)2.5 Ci
C)3.0 Ci
D)3.5 Ci
E)4.0 Ci

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
24
Which one of the following statements is true concerning the radioisotope carbon-14 that is used in carbon dating?
A)Carbon-14 is produced by living cells.
B)Carbon-14 is produced during - decay.
C)Carbon-14 is produced by the decay of carbon-12.
D)Carbon-14 is produced by cells after they have died.
E)Carbon-14 is produced by cosmic rays striking the atmosphere.
A)Carbon-14 is produced by living cells.
B)Carbon-14 is produced during - decay.
C)Carbon-14 is produced by the decay of carbon-12.
D)Carbon-14 is produced by cells after they have died.
E)Carbon-14 is produced by cosmic rays striking the atmosphere.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
25
The same activity is measured for two different isotope samples. One sample contains 0.0450 kg of 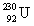 (atomic mass = 230.033 937 u, t1/2 = 20.8 days). The second sample contains an unknown amount of
(atomic mass = 230.033 937 u, t1/2 = 20.8 days). The second sample contains an unknown amount of 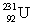 (atomic mass = 231.036 264 u, t1/2 = 4.3 days). What is the mass of the second sample?
(atomic mass = 231.036 264 u, t1/2 = 4.3 days). What is the mass of the second sample?
A)0.0093 kg
B)0.23 kg
C)0.110 kg
D)0.037 kg
E)0.0450 kg
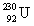 (atomic mass = 230.033 937 u, t1/2 = 20.8 days). The second sample contains an unknown amount of
(atomic mass = 230.033 937 u, t1/2 = 20.8 days). The second sample contains an unknown amount of 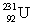 (atomic mass = 231.036 264 u, t1/2 = 4.3 days). What is the mass of the second sample?
(atomic mass = 231.036 264 u, t1/2 = 4.3 days). What is the mass of the second sample?A)0.0093 kg
B)0.23 kg
C)0.110 kg
D)0.037 kg
E)0.0450 kg

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
26
If the uranium nucleus is at rest before its decay, which one of the following statements is true concerning the final nuclei?
A)They have equal kinetic energies, but the thorium nucleus has much more momentum.
B)They have momenta of equal magnitudes, but the thorium nucleus has much more kinetic energy.
C)They have equal kinetic energies and momenta of equal magnitudes.
D)They have equal kinetic energies, but X has much more momentum.
E)They have momenta of equal magnitudes, but X has much more kinetic energy.
A)They have equal kinetic energies, but the thorium nucleus has much more momentum.
B)They have momenta of equal magnitudes, but the thorium nucleus has much more kinetic energy.
C)They have equal kinetic energies and momenta of equal magnitudes.
D)They have equal kinetic energies, but X has much more momentum.
E)They have momenta of equal magnitudes, but X has much more kinetic energy.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
27
An isotope of krypton has a half-life of 3 minutes. A sample of this isotope produces 1000 counts per minute in a Geiger counter. Determine the number of counts per minute produced after 15 minutes.
A)zero
B)15
C)30
D)60
E)1000
A)zero
B)15
C)30
D)60
E)1000

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
28
The ratio of the abundance of carbon-14 to carbon-12 in a sample of dead wood is one quarter the ratio for living wood. If the half-life of carbon-14 is 5730 years, which one of the following expressions determines how many years ago the wood died?
A)2 × 5730
B)4 × 5730
C)0.75 × 5730
D)0.50 × 5730
E)0.25 × 5730
A)2 × 5730
B)4 × 5730
C)0.75 × 5730
D)0.50 × 5730
E)0.25 × 5730

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
29
Tritium is an isotope of hydrogen that has two neutrons in addition to its proton. Tritium undergoes - decay with a half-life of 12.3 years. What percentage of an initially pure sample of tritium will remain undecayed after 35 years?
A)2.9 %
B)6.0 %
C)7.0 %
D)14 %
E)19 %
A)2.9 %
B)6.0 %
C)7.0 %
D)14 %
E)19 %

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
30
What is X?
A)
B)p
C) +
D) -
E)n
A)
B)p
C) +
D) -
E)n

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
31
Determine the amount of energy released in this decay. Use the following atomic masses: 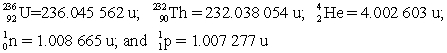 Conversion factors: 1 u = 931.5 MeV; 1 eV = 1.602 × 10-19 J
Conversion factors: 1 u = 931.5 MeV; 1 eV = 1.602 × 10-19 J
A)3.5 × 10-8 J
B)6.0 × 10-10 J
C)4.6 × 10-12 J
D)7.3 × 10-13 J
E)2.9 × 10-12 J
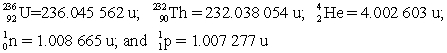 Conversion factors: 1 u = 931.5 MeV; 1 eV = 1.602 × 10-19 J
Conversion factors: 1 u = 931.5 MeV; 1 eV = 1.602 × 10-19 JA)3.5 × 10-8 J
B)6.0 × 10-10 J
C)4.6 × 10-12 J
D)7.3 × 10-13 J
E)2.9 × 10-12 J

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
32
The half-life of a particular isotope of iodine is 8.0 days. How much of a 10.0-g sample of this isotope will remain after 30 days?
A)0.37 g
B)0.45 g
C)0.60 g
D)0.74 g
E)1.25 g
A)0.37 g
B)0.45 g
C)0.60 g
D)0.74 g
E)1.25 g

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
33
Determine the amount of energy released in the following alpha decay process: 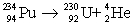 The relevant atomic masses are
The relevant atomic masses are 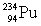 = 234.043 299 u,
= 234.043 299 u, 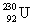 =230.033 937 u, and
=230.033 937 u, and 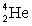 = 4.002 603 u.
= 4.002 603 u.
A)3.73 keV
B)927 keV
C)6.30 MeV
D)8.04 MeV
E)10.6 MeV
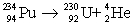 The relevant atomic masses are
The relevant atomic masses are 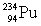 = 234.043 299 u,
= 234.043 299 u, 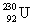 =230.033 937 u, and
=230.033 937 u, and 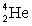 = 4.002 603 u.
= 4.002 603 u.A)3.73 keV
B)927 keV
C)6.30 MeV
D)8.04 MeV
E)10.6 MeV

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
34
The half-life a particular isotope of barium is 12 s. What is the activity of a 1.0 × 10-6 kg sample of this isotope?
A)1.2 × 1015 Bq
B)1.8 × 1016 Bq
C)2.5 × 1017 Bq
D)3.6 × 1018 Bq
E)6.0 × 1023 Bq
A)1.2 × 1015 Bq
B)1.8 × 1016 Bq
C)2.5 × 1017 Bq
D)3.6 × 1018 Bq
E)6.0 × 1023 Bq

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
35
In a certain decay process, an excited neon atom emits a -ray that has an energy of 1.630 MeV. The neon atom in the ground state has a mass of 19.992 435 u. What is the mass of the excited neon atom?
A)17.498 658 u
B)18.746 422 u
C)19.994 185 u
D)19.999 685 u
E)20.003 185 u
A)17.498 658 u
B)18.746 422 u
C)19.994 185 u
D)19.999 685 u
E)20.003 185 u

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
36
The activity of a carbon-14 sample is 0.1 Ci. If this sample is burned, what is the activity of the resulting CO2?
A)zero curies
B)0.1 Ci
C)0.2 Ci
D)0.3 Ci
E)2.00 Ci
A)zero curies
B)0.1 Ci
C)0.2 Ci
D)0.3 Ci
E)2.00 Ci

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
37
A sample contains 1000 nuclei of a radioactive isotope of barium. Exactly sixty seconds later, 970 nuclei in the sample have decayed. Determine the half-life of this isotope.
A)10 s
B)12 s
C)14 s
D)16 s
E)18 s
A)10 s
B)12 s
C)14 s
D)16 s
E)18 s

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck


