Exam 31: Nuclear Physics and Radioactivity
Exam 1: Introduction and Mathematical Concepts70 Questions
Exam 2: Kinematics in One Dimension103 Questions
Exam 3: Kinematics in Two Dimensions68 Questions
Exam 4: Forces and Newtons Laws of Motion103 Questions
Exam 5: Dynamics of Uniform Circular Motion59 Questions
Exam 6: Work and Energy78 Questions
Exam 7: Impulse and Momentum66 Questions
Exam 8: Rotational Kinematics55 Questions
Exam 9: Rotational Dynamics57 Questions
Exam 10: Simple Harmonic Motion and Elasticity63 Questions
Exam 11: Fluids65 Questions
Exam 12: Temperature and Heat66 Questions
Exam 13: The Transfer of Heat42 Questions
Exam 14: The Ideal Gas Law and Kinetic Theory55 Questions
Exam 15: Thermodynamics79 Questions
Exam 16: Waves and Sound67 Questions
Exam 17: The Principle of Linear Superposition and Interference Phenomena46 Questions
Exam 18: Electric Forces and Electric Fields61 Questions
Exam 19: Electric Potential Energy and the Electric Potential70 Questions
Exam 20: Electric Circuits100 Questions
Exam 21: Magnetic Forces and Magnetic Fields66 Questions
Exam 22: Electromagnetic Induction71 Questions
Exam 23: Alternating Current Circuits84 Questions
Exam 24: Electromagnetic Waves66 Questions
Exam 25: The Refl Ection of Light: Mirrors43 Questions
Exam 26: The Refraction of Light: Lenses and Optical Instruments102 Questions
Exam 27: Interference and the Wave Nature of Light57 Questions
Exam 28: Special Relativity63 Questions
Exam 29: Particles and Waves54 Questions
Exam 30: The Nature of the Atom74 Questions
Exam 31: Nuclear Physics and Radioactivity37 Questions
Exam 32: Ionizing Radiation, Nuclear Energy, and Elementary Particles45 Questions
Select questions type
Which one of the following statements concerning stable nuclei is true?
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
E
Which process is involved in determining the age of a prehistoric object?
Free
(Multiple Choice)
4.7/5  (33)
(33)
Correct Answer:
B
Which one of the following descriptive terms does not apply to nuclear forces?
Free
(Multiple Choice)
4.9/5  (30)
(30)
Correct Answer:
E
The half-life a particular isotope of barium is 12 s. What is the activity of a 1.0 × 10-6 kg sample of this isotope?
(Multiple Choice)
4.8/5  (37)
(37)
Tritium is an isotope of hydrogen that has two neutrons in addition to its proton. Tritium undergoes - decay with a half-life of 12.3 years. What percentage of an initially pure sample of tritium will remain undecayed after 35 years?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following is not an assumption involved in the expression: r = (1.2 × 10-15 m) A1/3?
(Multiple Choice)
4.9/5  (39)
(39)
Determine the amount of energy released in this decay. Use the following atomic masses: 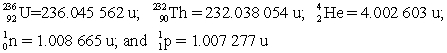 Conversion factors: 1 u = 931.5 MeV; 1 eV = 1.602 × 10-19 J
Conversion factors: 1 u = 931.5 MeV; 1 eV = 1.602 × 10-19 J
(Multiple Choice)
4.9/5  (26)
(26)
The activity of carbon-14 in a sample of charcoal from an archaeological site is 0.04 Bq. Determine the age of the sample. The half-life of carbon-14 is 5730 years.
(Multiple Choice)
4.7/5  (35)
(35)
The half-life of a particular isotope of iodine is 8.0 days. How much of a 10.0-g sample of this isotope will remain after 30 days?
(Multiple Choice)
4.8/5  (38)
(38)
This question refers to the figure shown. Which one of the following concepts explains why heavy nuclei do not follow the N = Z line (or trend) in the figure? 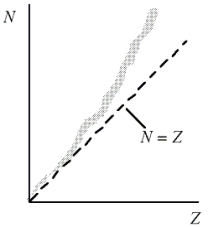
(Multiple Choice)
4.8/5  (36)
(36)
The ratio of the abundance of carbon-14 to carbon-12 in a sample of dead wood is one quarter the ratio for living wood. If the half-life of carbon-14 is 5730 years, which one of the following expressions determines how many years ago the wood died?
(Multiple Choice)
4.8/5  (33)
(33)
Which one of the following statements is true concerning the radioisotope carbon-14 that is used in carbon dating?
(Multiple Choice)
4.9/5  (32)
(32)
What is the wavelength of the 0.059-MeV ray photon emitted by 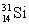 ?
?
(Multiple Choice)
4.9/5  (36)
(36)
Radiation or(and) particles emerge(s) from a radioactive sample. These products from the sample are allowed to pass through a narrow slit and may be considered a beam. The beam is passed between two plates that carry opposite electrical charge. The experimental region contains no magnetic fields. It is observed that the beam is deflected toward the negatively charged plate. 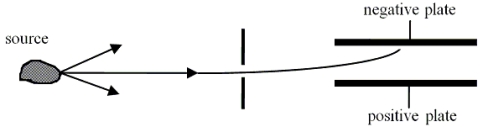 Which one of the following statements is the best conclusion for this situation?
Which one of the following statements is the best conclusion for this situation?
(Multiple Choice)
4.9/5  (32)
(32)
An isotope of krypton has a half-life of 3 minutes. A sample of this isotope produces 1000 counts per minute in a Geiger counter. Determine the number of counts per minute produced after 15 minutes.
(Multiple Choice)
4.8/5  (32)
(32)
Complete the following sentence: In a + decay process, the emitted particle is
(Multiple Choice)
4.8/5  (29)
(29)
The same activity is measured for two different isotope samples. One sample contains 0.0450 kg of 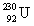 (atomic mass = 230.033 937 u, t1/2 = 20.8 days). The second sample contains an unknown amount of
(atomic mass = 230.033 937 u, t1/2 = 20.8 days). The second sample contains an unknown amount of 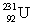 (atomic mass = 231.036 264 u, t1/2 = 4.3 days). What is the mass of the second sample?
(atomic mass = 231.036 264 u, t1/2 = 4.3 days). What is the mass of the second sample?
(Multiple Choice)
4.9/5  (34)
(34)
Osmium has atomic number 76. A particular isotope of osmium has an atomic mass of 191.961 u. Which symbol correctly represents this isotope?
(Multiple Choice)
4.8/5  (28)
(28)
Showing 1 - 20 of 37
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)