Deck 6: Liquids and Solids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/94
Play
Full screen (f)
Deck 6: Liquids and Solids
1
What are all the intermolecular forces that are responsible for the existence of ice?
A)Dipole-dipole and London forces
B)London forces
C)Dipole-dipole, London forces, and hydrogen bonding
D)Dipole-dipole and ion-ion
E)Hydrogen bonding and dipole-dipole
A)Dipole-dipole and London forces
B)London forces
C)Dipole-dipole, London forces, and hydrogen bonding
D)Dipole-dipole and ion-ion
E)Hydrogen bonding and dipole-dipole
Dipole-dipole, London forces, and hydrogen bonding
2
The compound KCN·2H2O is likely to be commonly available and thus found in the laboratory.True or false?
False
3
Which has the higher boiling point,1,1-dichloroethene or cis-dichloroethene?
1,1-dichloroethene
4
Which has the higher boiling point,HCl or HI?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following can form intermolecular hydrogen bonds?
A)NH2CH2COOH
B)SiH4
C)CH3COCH3
D)H2CO
E)CH3Cl
A)NH2CH2COOH
B)SiH4
C)CH3COCH3
D)H2CO
E)CH3Cl

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following can form intermolecular hydrogen bonds?
A)CH3CH2CH2CH2CH3
B)CH3CH2C(O)H
C)CH3CH2C(O)CH3
D)CH3CH2NH2
E)CH3CH2OCH2CH3
A)CH3CH2CH2CH2CH3
B)CH3CH2C(O)H
C)CH3CH2C(O)CH3
D)CH3CH2NH2
E)CH3CH2OCH2CH3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following cations is likely to be hydrated in compounds?
A)Rb+
B)NH4+
C)Ba2+
D)K+
E)Cs+
A)Rb+
B)NH4+
C)Ba2+
D)K+
E)Cs+

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following has the lowest boiling point?
A)HF
B)GeH4
C)SiH4
D)PH3
E)H2Se
A)HF
B)GeH4
C)SiH4
D)PH3
E)H2Se

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following has the highest boiling point?
A)N2
B)H2S
C)NH3
D)H2O
E)SO2
A)N2
B)H2S
C)NH3
D)H2O
E)SO2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements is true?
A)Butane, C4H10, has a higher boiling point than acetone, CH3COCH3.
B)Pentane, C5H12, has a lower boiling point than 2,2-dimethylpropane, C5H12.
C)CHF3 has a higher boiling point than CF4.
D)CH4 has a higher boiling point than CCl4.
E)HI has a lower boiling point than HBr.
A)Butane, C4H10, has a higher boiling point than acetone, CH3COCH3.
B)Pentane, C5H12, has a lower boiling point than 2,2-dimethylpropane, C5H12.
C)CHF3 has a higher boiling point than CF4.
D)CH4 has a higher boiling point than CCl4.
E)HI has a lower boiling point than HBr.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is the strongest intermolecular force between molecules?
A)Dipole-dipole (stationary)
B)Hydrogen bonding
C)Dipole-dipole (rotating)
D)London
E)Ion-dipole
A)Dipole-dipole (stationary)
B)Hydrogen bonding
C)Dipole-dipole (rotating)
D)London
E)Ion-dipole

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
12
If the interaction between two species is proportional to 1/r6,which of the following is likely involved?
A)Chloromethane molecules in the gas phase
B)Chloromethane molecules in the solid phase
C)Li+ and H2O
D)Na+ and H2O
E)Ions in an ionic solid
A)Chloromethane molecules in the gas phase
B)Chloromethane molecules in the solid phase
C)Li+ and H2O
D)Na+ and H2O
E)Ions in an ionic solid

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following cations is likely to be hydrated in compounds?
A)Cs+
B)NH4+
C)La3+
D)Rb+
E)K+
A)Cs+
B)NH4+
C)La3+
D)Rb+
E)K+

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following statements is true?
A)1,1-Dichloro-2-methyl-1-propene has a higher boiling point than trans-2,3-dichloro-2-butene.
B)CH4 has a higher boiling point than CCl4.
C)o-Dichlorobenzene has a lower boiling point than p-dichlorobenzene.
D)HI has a lower boiling point than HBr.
E)Butane, C4H10, has a higher boiling point than acetone, CH3COCH3.
A)1,1-Dichloro-2-methyl-1-propene has a higher boiling point than trans-2,3-dichloro-2-butene.
B)CH4 has a higher boiling point than CCl4.
C)o-Dichlorobenzene has a lower boiling point than p-dichlorobenzene.
D)HI has a lower boiling point than HBr.
E)Butane, C4H10, has a higher boiling point than acetone, CH3COCH3.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following has the highest boiling point?
A)CH3CH2CH2CH2CH2Br
B)CH3CH2CH2CH2CH2I
C)CH3CH2CH2CH2CH3
D)CH3CH2CH2CH2CH2F
E)CH3CH2CH2CH2CH2Cl
A)CH3CH2CH2CH2CH2Br
B)CH3CH2CH2CH2CH2I
C)CH3CH2CH2CH2CH3
D)CH3CH2CH2CH2CH2F
E)CH3CH2CH2CH2CH2Cl

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
16
If the interaction between two species is proportional to 1/r2,which of the following is likely involved?
A)Chloromethane molecules in the liquid phase
B)Na+ and H2O
C)Bromine molecules in the liquid phase
D)Chloromethane molecules in the gas phase
E)Ions in an ionic solid
A)Chloromethane molecules in the liquid phase
B)Na+ and H2O
C)Bromine molecules in the liquid phase
D)Chloromethane molecules in the gas phase
E)Ions in an ionic solid

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
17
If the interaction between two species is proportional to 1/r3,which of the following is likely involved?
A)Chloromethane molecules in the liquid phase
B)Ions in an ionic solid
C)Bromine molecules in the liquid phase
D)Chloromethane molecules in the solid phase
E)Na+ and H2O
A)Chloromethane molecules in the liquid phase
B)Ions in an ionic solid
C)Bromine molecules in the liquid phase
D)Chloromethane molecules in the solid phase
E)Na+ and H2O

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
18
All the following hydrated compounds are commonly available except
A)BaCl2·2H2O.
B)NH4NO3·2H2O.
C)NaClO4·H2O.
D)Cr(ClO4)3·6H2O.
E)La2(SO4)3·9H2O.
A)BaCl2·2H2O.
B)NH4NO3·2H2O.
C)NaClO4·H2O.
D)Cr(ClO4)3·6H2O.
E)La2(SO4)3·9H2O.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following cations is likely to be hydrated in compounds?
A)Rb+
B)Li+
C)K+
D)Cs+
E)NH4+
A)Rb+
B)Li+
C)K+
D)Cs+
E)NH4+

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following can form intermolecular hydrogen bonds?
A)PH3
B)HI
C)CH3CH2CH2CH2CH3
D)HCOOH
E)CH3CH2OCH2CH3
A)PH3
B)HI
C)CH3CH2CH2CH2CH3
D)HCOOH
E)CH3CH2OCH2CH3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
21
How many tetrahedral and octahedral holes per atom are there in a cubic close-packed structure,respectively?
A)2 and 1
B)0 and 1
C)2 and 2
D)2 and 0
E)0 and 2
A)2 and 1
B)0 and 1
C)2 and 2
D)2 and 0
E)0 and 2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
22
The coordination numbers in cubic close-packed,body-centered cubic,and primitive cubic structures are,respectively,
A)12, 8, and 12.
B)12, 8, and 4.
C)8, 12, and 6.
D)12, 6, and 6.
E)12, 8, and 6.
A)12, 8, and 12.
B)12, 8, and 4.
C)8, 12, and 6.
D)12, 6, and 6.
E)12, 8, and 6.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
23
A metal with a cubic close-packed structure is malleable because
A)Each atom has 2 tetrahedral holes.
B)Each atom has a coordination number of 12.
C)The unit cell has 8 sets of slip planes.
D)The unit cell has a large percentage of empty space.
E)Each atom has 1 octahedral hole.
A)Each atom has 2 tetrahedral holes.
B)Each atom has a coordination number of 12.
C)The unit cell has 8 sets of slip planes.
D)The unit cell has a large percentage of empty space.
E)Each atom has 1 octahedral hole.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following molecular solids has the highest melting point?
A)Sucrose, C12H22O11
B)Carbon dioxide, CO2
C)H2O
D)TiCl4
E)Benzene, C6H6
A)Sucrose, C12H22O11
B)Carbon dioxide, CO2
C)H2O
D)TiCl4
E)Benzene, C6H6

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
25
Determine the percentage of the total volume that is empty space in a primitive cubic structure in which all the atoms are identical.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
26
A cubic close-packed structure has
A)A coordination number of 4.
B)A coordination number of 6.
C)The same density as a hexagonal close-packed structure.
D)Twice the density of a hexagonal close-packed structure.
E)A coordination number of 8.
A)A coordination number of 4.
B)A coordination number of 6.
C)The same density as a hexagonal close-packed structure.
D)Twice the density of a hexagonal close-packed structure.
E)A coordination number of 8.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
27
Water was found to rise to a height of 7.4 mm in a tube of internal radius 2.0 mm at room temperature.Given its density as 1.0 gcm3,what is the surface tension of water?
A)0.15 Nm1
B)0.036 Nm1
C)7.3 10-8 Nm1
D)0.073 Nm1
E)1.5 10-7 Nm1
A)0.15 Nm1
B)0.036 Nm1
C)7.3 10-8 Nm1
D)0.073 Nm1
E)1.5 10-7 Nm1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
28
How many atoms are there in a face-centered cubic unit cell?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
29
Predict which of the following has the lowest boiling point.
A)SbH3
B)AsH3
C)NH3
D)BiH3
E)PH3
A)SbH3
B)AsH3
C)NH3
D)BiH3
E)PH3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
30
Butane and 2-propanone have approximately the same boiling points.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
31
Predict which of the following liquids has the lowest enthalpy of vaporization.
A)H2Se
B)H2O
C)H2Po
D)H2S
E)H2Te
A)H2Se
B)H2O
C)H2Po
D)H2S
E)H2Te

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
32
Viscosity usually decreases with increasing temperature.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
33
Pentane and 2,2-dimethylpropane have the formula C5H12 and therefore have the same boiling point.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
34
Glycerol,C3H8O3,has a higher viscosity than propanol,C3H8O.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
35
How many atoms are there in a primitive cubic unit cell?
A)1
B)2
C)3
D)8
E)4
A)1
B)2
C)3
D)8
E)4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
36
Tetrabromomethane has a higher boiling point than tetrachloromethane.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
37
All the following are units of surface tension except
A)mJm2.
B)Nm.
C)Jm2.
D)mNm1.
E)kgs2.
A)mJm2.
B)Nm.
C)Jm2.
D)mNm1.
E)kgs2.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
38
What are all the intermolecular forces that are responsible for the existence of the molecular solid oxalic acid,H2C2O4?
A)Dipole-dipole, London forces, and hydrogen bonding
B)Dipole-dipole and London forces
C)Hydrogen bonding and dipole-dipole
D)London forces
E)Dipole-dipole and ion-ion
A)Dipole-dipole, London forces, and hydrogen bonding
B)Dipole-dipole and London forces
C)Hydrogen bonding and dipole-dipole
D)London forces
E)Dipole-dipole and ion-ion

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
39
The mass of a face-centered cubic unit cell is
A)Two times the mass of one atom.
B)Five times the mass of one atom.
C)Equal to the mass of one atom.
D)Six times the mass of one atom.
E)Four times the mass of one atom.
A)Two times the mass of one atom.
B)Five times the mass of one atom.
C)Equal to the mass of one atom.
D)Six times the mass of one atom.
E)Four times the mass of one atom.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
40
The ability of water to "wet" paper is due to hydrogen bonding between water molecules and surface molecules in the paper.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
41
Which answer best accounts for the Cl ions present in the NaCl unit cell?
A)1 center atom + 1/2 6 face atoms
B)1/8 8 corner atoms + 1/2 6 face atoms
C)1 center atom + 1/8 8 corner atoms
D)1 center atom + 1/4 12 edge atoms
E)1 center atom + 1/8 24 corner atoms
A)1 center atom + 1/2 6 face atoms
B)1/8 8 corner atoms + 1/2 6 face atoms
C)1 center atom + 1/8 8 corner atoms
D)1 center atom + 1/4 12 edge atoms
E)1 center atom + 1/8 24 corner atoms

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
42
The atomic radius of aluminum is 143 pm.Estimate its density,given that the metal has a close-packed structure.
A)3.87 gcm3
B)5.54 gcm3
C)7.92 gcm3
D)16.3 gcm3
E)2.71 gcm3
A)3.87 gcm3
B)5.54 gcm3
C)7.92 gcm3
D)16.3 gcm3
E)2.71 gcm3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
43
How many calcium,titanium,and oxygen ions are there in the perovskite unit cell shown below? 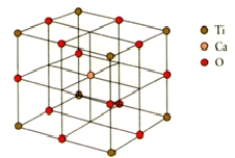
A)1 Ca2+, 2 Ti4+, and 3 O2
B)1 Ca2+, 1 Ti4+, and 6 O2
C)1 Ca2+, 4 Ti4+, and 6 O2
D)1 Ca2+, 1 Ti4+, and 3 O2
E)1 Ca2+, 2 Ti4+, and 6 O2

A)1 Ca2+, 2 Ti4+, and 3 O2
B)1 Ca2+, 1 Ti4+, and 6 O2
C)1 Ca2+, 4 Ti4+, and 6 O2
D)1 Ca2+, 1 Ti4+, and 3 O2
E)1 Ca2+, 2 Ti4+, and 6 O2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
44
What are the coordination numbers of Ca2+ and F ions,respectively,in fluorite? The unit cell is shown below 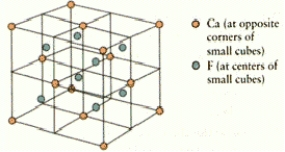
A)6 and 8
B)4 and 8
C)8 and 8
D)6 and 4
E)8 and 4

A)6 and 8
B)4 and 8
C)8 and 8
D)6 and 4
E)8 and 4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
45
The atomic radius of zinc is 137 pm.Estimate its density,given that the metal has a close-packed structure.
A)7.47 gcm3
B)10.2 gcm3
C)14.0 gcm3
D)4.49 gcm3
E)19.2 gcm3
A)7.47 gcm3
B)10.2 gcm3
C)14.0 gcm3
D)4.49 gcm3
E)19.2 gcm3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
46
The density of solid krypton is 2.16 gcm3.Krypton crystallizes in a cubic close-packed structure; estimate the atomic radius.
A)66.5 pm
B)225 pm
C)113 pm
D)318 pm
E)450 pm
A)66.5 pm
B)225 pm
C)113 pm
D)318 pm
E)450 pm

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
47
Estimate the density of cesium iodide from its crystal structure.The ionic radii of Cs+ and Cl are 170 and 220 pm,respectively.
A)7.25 gcm3
B)18.9 gcm3
C)9.44 gcm3
D)4.72 gcm3
E)3.77 gcm3
A)7.25 gcm3
B)18.9 gcm3
C)9.44 gcm3
D)4.72 gcm3
E)3.77 gcm3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
48
How many calcium and fluorine ions are there in the fluorite unit cell shown below? 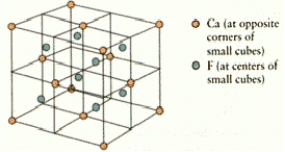
A)4 Ca2+ and 2 F
B)4 Ca2+ and 8 F
C)2 Ca2+ and 4 F
D)2 Ca2+ and 8 F
E)4 Ca2+ and 4 F

A)4 Ca2+ and 2 F
B)4 Ca2+ and 8 F
C)2 Ca2+ and 4 F
D)2 Ca2+ and 8 F
E)4 Ca2+ and 4 F

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
49
What is the formula of the superconductor whose unit cell is shown below? (The Y and Ba atoms are in the middle of the cell.) 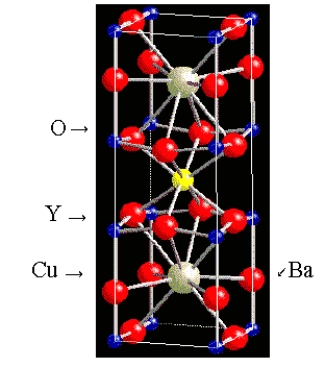
A)YBa2Cu3O9
B)YBa2Cu4O5
C)YBa2Cu2O7
D)YBa2Cu4O7
E)YBa2Cu3O7

A)YBa2Cu3O9
B)YBa2Cu4O5
C)YBa2Cu2O7
D)YBa2Cu4O7
E)YBa2Cu3O7

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
50
Estimate the density of magnesium oxide from its crystal structure.The radii of Mg2+ and O2 are 72 and 140 pm,respectively.
A)3.51 gcm3
B)14.9 gcm3
C)21.1 gcm3
D)1.76 gcm3
E)0.878 gcm3
A)3.51 gcm3
B)14.9 gcm3
C)21.1 gcm3
D)1.76 gcm3
E)0.878 gcm3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
51
The atomic radius of magnesium is 160 pm.Estimate its density,given that the metal has a close-packed structure.
A)1.74 gcm3
B)0.435 gcm3
C)10.5 gcm3
D)4.45 gcm3
E)2.78 gcm3
A)1.74 gcm3
B)0.435 gcm3
C)10.5 gcm3
D)4.45 gcm3
E)2.78 gcm3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
52
If the radius of an atom is r,what is the length of the side of the body-centered cubic unit cell?
A)4r/3½
B)2.25r
C)r
D)2r
E)8½r
A)4r/3½
B)2.25r
C)r
D)2r
E)8½r

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
53
If the radius of an atom is r,what is the length of the side of the face-centered cubic unit cell?
A)8½r
B)2.25r
C)2r
D)4r/3½
E)r
A)8½r
B)2.25r
C)2r
D)4r/3½
E)r

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
54
Molecular solids are held together by weak intermolecular forces.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
55
How many titanium and oxygen ions are there in the rutile unit cell shown below? 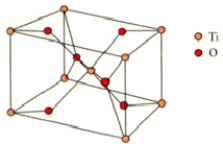
A)3 Ti4+ and 4 O2
B)3 Ti4+ and 6 O2
C)2 Ti4+ and 4 O2
D)2 Ti4+ and 3 O2
E)2 Ti4+ and 6 O2

A)3 Ti4+ and 4 O2
B)3 Ti4+ and 6 O2
C)2 Ti4+ and 4 O2
D)2 Ti4+ and 3 O2
E)2 Ti4+ and 6 O2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
56
The density of sodium metal is 0.97 gcm3.Sodium crystallizes in a body-centered cubic structure; estimate the atomic radius.
A)95 pm
B)320 pm
C)370 pm
D)65 pm
E)190 pm
A)95 pm
B)320 pm
C)370 pm
D)65 pm
E)190 pm

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
57
All the elements listed below can exist as network solids except
A)O.
B)Si.
C)B.
D)As.
E)C.
A)O.
B)Si.
C)B.
D)As.
E)C.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
58
What are the coordination numbers of Ti4+ and O2,respectively,in rutile? The unit cell is shown below. 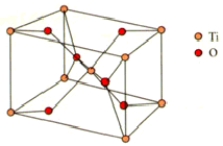
A)4 and 3
B)6 and 4
C)3 and 6
D)6 and 6
E)6 and 3

A)4 and 3
B)6 and 4
C)3 and 6
D)6 and 6
E)6 and 3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
59
Which answer best accounts for the Na+ ions present in the NaCl unit cell?
A)1 center atom + 1/8 24 corner atoms
B)1 center atom + 1/2 6 face atoms
C)1 center atom + 1/8 8 corner atoms
D)1 center atom + 1/4 12 edge atoms
E)1/8 8 corner atoms + 1/2 6 face atoms
A)1 center atom + 1/8 24 corner atoms
B)1 center atom + 1/2 6 face atoms
C)1 center atom + 1/8 8 corner atoms
D)1 center atom + 1/4 12 edge atoms
E)1/8 8 corner atoms + 1/2 6 face atoms

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
60
What are the coordination numbers of calcium and titanium,respectively,in perovskite? The unit cell is shown below. 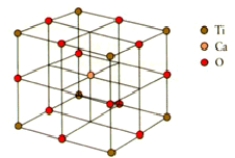
A)6 and 6
B)12 and 6
C)12 and 8
D)12 and 12
E)6 and 8

A)6 and 6
B)12 and 6
C)12 and 8
D)12 and 12
E)6 and 8

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
61
An amorphous solid is one in which the atoms,ions,or molecules lie in a random jumble with no order.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
62
The solid ZnS has a radius ratio of 0.45 and adopts the zinc-blende structure.What is the coordination number of Zn in ZnS?
A)12
B)4
C)8
D)2
E)6
A)12
B)4
C)8
D)2
E)6

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
63
What is the coordination number of rubidium in RbF? The ionic radii of Rb+ and F are 149 and 133 pm,respectively.
A)2
B)4
C)8
D)6
E)12
A)2
B)4
C)8
D)6
E)12

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
64
Calculate the size of the octahedral holes in the NaCl structure.The ionic radii of Na+ and Cl are 102 and 181 pm,respectively.
A)142 pm
B)181 pm
C)102 pm
D)91 pm
E)75 pm
A)142 pm
B)181 pm
C)102 pm
D)91 pm
E)75 pm

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
65
What is the coordination number of cesium in CsCl? The ionic radii of Cs+ and Cl are 170 and 181 pm,respectively.
A)12
B)8
C)2
D)4
E)6
A)12
B)8
C)2
D)4
E)6

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
66
A certain liquid has a meniscus that curves downward in glass.This means that the cohesive forces in the liquid are less than the forces between the liquid and the glass.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following statements is true?
A)A metallic conductor is a substance with a resistance that increases with increasing temperature.
B)A superconductor is a substance that has zero resistance below a certain temperature.
C)An insulator is a substance that does not conduct electricity below a certain temperature.
D)A semiconductor is a substance with a resistance that increases with increasing temperature.
E)An insulator behaves like a metallic conductor with a very high resistance.
A)A metallic conductor is a substance with a resistance that increases with increasing temperature.
B)A superconductor is a substance that has zero resistance below a certain temperature.
C)An insulator is a substance that does not conduct electricity below a certain temperature.
D)A semiconductor is a substance with a resistance that increases with increasing temperature.
E)An insulator behaves like a metallic conductor with a very high resistance.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following are heterogeneous alloys?
A)Mercury amalgam and tin-lead solder
B)Brass
C)Bronze
D)Tin-lead solder and bronze
E)Coinage cupronickel
A)Mercury amalgam and tin-lead solder
B)Brass
C)Bronze
D)Tin-lead solder and bronze
E)Coinage cupronickel

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
69
How many tetrahedral holes are there in a face-centered cubic unit cell?
A)8
B)12
C)1
D)4
E)6
A)8
B)12
C)1
D)4
E)6

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
70
The boiling points of the Group 15 binary hydrides increase smoothly from NH3 to SbH3.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
71
What is the coordination number of silver in AgCl? The ionic radii of Ag+ and Cl are 113 and 181 pm,respectively.
A)4
B)2
C)6
D)8
E)12
A)4
B)2
C)6
D)8
E)12

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
72
The boiling points of the Group 14 binary hydrides increase smoothly from CH4 to SnH4.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following liquids has the lowest viscosity?
A)Acetone
B)Ethanol
C)Phosphoric acid
D)Benzene
A)Acetone
B)Ethanol
C)Phosphoric acid
D)Benzene

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
74
Metals with a hexagonal close-packed structure have/do not have slip planes and as a result are malleable/brittle.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
75
How many octahedral holes are there in a face-centered cubic unit cell?
A)6
B)13
C)12
D)4
E)8
A)6
B)13
C)12
D)4
E)8

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
76
If the ratio of the radius of the cation to the anion in a 1:1 compound is 0.800,
A)The compound adopts the rock-salt structure.
B)The coordination number of the cation and anion are 6 and 6.
C)The compound adopts the same structure as calcium fluorite.
D)Each cation occupies one-half of the tetrahedral holes of each anion cube.
E)Each cation occupies all the cubic holes in the center of each anion cube.
A)The compound adopts the rock-salt structure.
B)The coordination number of the cation and anion are 6 and 6.
C)The compound adopts the same structure as calcium fluorite.
D)Each cation occupies one-half of the tetrahedral holes of each anion cube.
E)Each cation occupies all the cubic holes in the center of each anion cube.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
77
Both ZnS and CaF2 have a face-centered cubic unit cell where the S2 and Ca2+ ions are closest packed in each structure.Which of the following is true?
A)There are 4 tetrahedral holes empty in each structure.
B)In both compounds, one-half of the tetrhedral holes are filled.
C)In both compounds, all the tetrhedral holes are filled.
D)In ZnS, one-half of the tetrahedral holes are filled by Zn2+ ions, whereas in CaF2 all the tetrahedral holes are filled with F ions.
E)There are 8 Zn2+ ions and 4 F ions in the unit cell.
A)There are 4 tetrahedral holes empty in each structure.
B)In both compounds, one-half of the tetrhedral holes are filled.
C)In both compounds, all the tetrhedral holes are filled.
D)In ZnS, one-half of the tetrahedral holes are filled by Zn2+ ions, whereas in CaF2 all the tetrahedral holes are filled with F ions.
E)There are 8 Zn2+ ions and 4 F ions in the unit cell.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
78
In the zinc-blende structure (ZnS),the cations occupy half the tetrahedral holes.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
79
What is the coordination number of magnesium in MgO? The ionic radii of Mg2+ and O2 are 72 and 140 pm,respectively.
A)4
B)2
C)8
D)6
E)12
A)4
B)2
C)8
D)6
E)12

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
80
Superconductivity is the loss of all electrical resistance when a substance is cooled below a certain characteristic transition temperature.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck


