Exam 6: Liquids and Solids
Exam 1: The Quantum World99 Questions
Exam 2: Quantum Mechanics in Action: Atoms100 Questions
Exam 3: Chemical Bonds84 Questions
Exam 4: Molecular Shape and Structure95 Questions
Exam 5: The Properties of Gases94 Questions
Exam 6: Liquids and Solids94 Questions
Exam 7: Inorganic Materials99 Questions
Exam 8: Thermodynamics: the First Law94 Questions
Exam 9: Thermodynamics: the Second and Third Laws93 Questions
Exam 10: Physical Equilibria94 Questions
Exam 11: Chemical Equilibria93 Questions
Exam 12: Acids and Bases94 Questions
Exam 13: Aqueous Equilibria94 Questions
Exam 14: Electrochemistry94 Questions
Exam 15: Chemical Kinetics88 Questions
Exam 16: The Elements: the Main Group Elements186 Questions
Exam 17: The Elements: the D Block93 Questions
Exam 18: Nuclear Chemistry95 Questions
Exam 19: Organic Chemistry I93 Questions
Exam 20: Organic Chemistry II94 Questions
Select questions type
How many calcium and fluorine ions are there in the fluorite unit cell shown below? 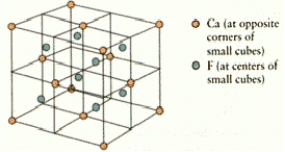
Free
(Multiple Choice)
4.7/5  (34)
(34)
Correct Answer:
B
Which has the higher boiling point,HCl or HI?
Free
(Short Answer)
4.9/5  (39)
(39)
Correct Answer:
HI
How many tetrahedral holes are there in a face-centered cubic unit cell?
Free
(Multiple Choice)
4.7/5  (34)
(34)
Correct Answer:
A
Which answer best accounts for the Cl ions present in the NaCl unit cell?
(Multiple Choice)
4.8/5  (28)
(28)
Molecular solids are held together by weak intermolecular forces.True or false?
(True/False)
4.9/5  (44)
(44)
The ability of water to "wet" paper is due to hydrogen bonding between water molecules and surface molecules in the paper.True or false?
(True/False)
5.0/5  (36)
(36)
The boiling points of the Group 15 binary hydrides increase smoothly from NH3 to SbH3.True or false?
(True/False)
4.8/5  (44)
(44)
The atomic radius of zinc is 137 pm.Estimate its density,given that the metal has a close-packed structure.
(Multiple Choice)
4.7/5  (47)
(47)
Pentane and 2,2-dimethylpropane have the formula C5H12 and therefore have the same boiling point.True or false?
(True/False)
4.9/5  (42)
(42)
All the elements listed below can exist as network solids except
(Multiple Choice)
4.9/5  (24)
(24)
What is the coordination number of cesium in CsCl? The ionic radii of Cs+ and Cl are 170 and 181 pm,respectively.
(Multiple Choice)
4.9/5  (40)
(40)
How many titanium and oxygen ions are there in the rutile unit cell shown below? 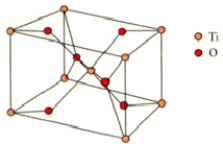
(Multiple Choice)
4.8/5  (33)
(33)
The solid ZnS has a radius ratio of 0.45 and adopts the zinc-blende structure.What is the coordination number of Zn in ZnS?
(Multiple Choice)
4.8/5  (38)
(38)
Determine the percentage of the total volume that is empty space in a primitive cubic structure in which all the atoms are identical.
(Short Answer)
4.8/5  (31)
(31)
Both ZnS and CaF2 have a face-centered cubic unit cell where the S2 and Ca2+ ions are closest packed in each structure.Which of the following is true?
(Multiple Choice)
4.8/5  (39)
(39)
What are all the intermolecular forces that are responsible for the existence of ice?
(Multiple Choice)
5.0/5  (45)
(45)
Estimate the density of magnesium oxide from its crystal structure.The radii of Mg2+ and O2 are 72 and 140 pm,respectively.
(Multiple Choice)
4.8/5  (46)
(46)
Showing 1 - 20 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)