Deck 12: Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/94
Play
Full screen (f)
Deck 12: Acids and Bases
1
Which of the following is the weakest acid?
A)HNO3
B)HBr
C)HCl
D)HF
E)HI
A)HNO3
B)HBr
C)HCl
D)HF
E)HI
HF
2
In the following reaction
SO2(g)+ H2O(l) H2SO3(aq),
identify the Lewis acid and base.
SO2(g)+ H2O(l) H2SO3(aq),
identify the Lewis acid and base.
Lewis acid,SO2; Lewis base,H2O.
3
When sulfur trioxide dissolves in water,sulfuric acid is produced.An intermediate in the reaction is H2O-SO3.In the reaction of the intermediate to produce sulfuric acid,
A)water acts both as an acid and a base.
B)water acts as a proton donor only.
C)water acts as a proton acceptor only.
D)the intermediate undergoes an intramolecular rearrangement to form the product.
A)water acts both as an acid and a base.
B)water acts as a proton donor only.
C)water acts as a proton acceptor only.
D)the intermediate undergoes an intramolecular rearrangement to form the product.
water acts both as an acid and a base.
4
Strong acids are leveled in water to the strength of the acid H3O+.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
5
The pH of a 0.0050 M aqueous solution of calcium hydroxide is
A)11.40
B)2.00
C)12.00
D)12.70
E)11.70
A)11.40
B)2.00
C)12.00
D)12.70
E)11.70

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
6
Calculate the hydroxide ion concentration for an aqueous solution that has a pH of 3.45.
A)3.2 102 M
B)0.54 M
C)3.5 104 M
D)2.8 1011 M
E)2.6 105 M
A)3.2 102 M
B)0.54 M
C)3.5 104 M
D)2.8 1011 M
E)2.6 105 M

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
7
The conjugate base of ammonia is
A)NH2OH.
B)NH2.
C)NH4+.
D)NH3.
E)OH.
A)NH2OH.
B)NH2.
C)NH4+.
D)NH3.
E)OH.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is the weakest acid?
A)HCN (pKa = 9.31)
B)HIO3 (pKa = 0.77)
C)HF (pKa = 3.45)
D)CH3COOH (pKa = 4.75)
E)HNO2 (pKa = 3.37)
A)HCN (pKa = 9.31)
B)HIO3 (pKa = 0.77)
C)HF (pKa = 3.45)
D)CH3COOH (pKa = 4.75)
E)HNO2 (pKa = 3.37)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
9
Of the following,which does not have an amphoteric oxide?
A)BeO
B)PbO
C)SnO
D)Al2O3
E)MgO
A)BeO
B)PbO
C)SnO
D)Al2O3
E)MgO

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
10
What is the pKa of the conjugate acid of hydrazine,given that the pKb of hydrazine is 5.77? Write the formula of the conjugate acid of hydrazine.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
11
What is the conjugate acid of O2?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
12
In a solution labeled "0.10 M HNO3," which of the following is correct?
A)[HNO3] = 0.10 M
B)[H3O+] = 0.10 M, [NO3] = 0.10 M
C)[H3O+] = 0.090 M, [NO3] = 0.010 M
D)[HNO3] = 0.050 M, [H3O+] = 0.050 M, [NO3] = 0.050 M
E)[H3O+] = 0.10 M, [OH] = 1.0 107 M
A)[HNO3] = 0.10 M
B)[H3O+] = 0.10 M, [NO3] = 0.10 M
C)[H3O+] = 0.090 M, [NO3] = 0.010 M
D)[HNO3] = 0.050 M, [H3O+] = 0.050 M, [NO3] = 0.050 M
E)[H3O+] = 0.10 M, [OH] = 1.0 107 M

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
13
Write the autoprotolysis reaction for liquid ammonia.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
14
What is the conjugate base of H2PO4 ?
A)HPO42
B)OH
C)H3PO4
D)H2PO4
E)PO43
A)HPO42
B)OH
C)H3PO4
D)H2PO4
E)PO43

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
15
All of the following are strong bases in water except
A)NaHCO3
B)CaO
C)Na2O
D)Na2SO4
A)NaHCO3
B)CaO
C)Na2O
D)Na2SO4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
16
The conjugate acid of HPO42 is
A)HPO42.
B)PO43.
C)H2PO4.
D)H3O+.
E)H3PO4.
A)HPO42.
B)PO43.
C)H2PO4.
D)H3O+.
E)H3PO4.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following produces the strongest conjugate base?
A)HF (pKa = 3.45)
B)HClO (pKa = 7.53)
C)HCOOH (pKa = 3.75)
D)CH3COOH (pKa = 4.75)
E)HIO (pKa = 10.64)
A)HF (pKa = 3.45)
B)HClO (pKa = 7.53)
C)HCOOH (pKa = 3.75)
D)CH3COOH (pKa = 4.75)
E)HIO (pKa = 10.64)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is the strongest base?
A)methylamine (pKb = 3.44)
B)morphine (pKb = 5.79)
C)urea (pKb = 13.90)
D)ammonia (pKb = 4.75)
E)pyridine (pKb = 8.75)
A)methylamine (pKb = 3.44)
B)morphine (pKb = 5.79)
C)urea (pKb = 13.90)
D)ammonia (pKb = 4.75)
E)pyridine (pKb = 8.75)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
19
Calculate the hydrogen ion concentration for an aqueous solution that has a pH of 3.45.
A)0.54 M
B)3.5 104 M
C)2.8 1011
D)3.2 102 M
E)1.22 M
A)0.54 M
B)3.5 104 M
C)2.8 1011
D)3.2 102 M
E)1.22 M

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
20
The conjugate base of OH is
A)H+.
B)OH.
C)O2.
D)H3O+.
E)H2O.
A)H+.
B)OH.
C)O2.
D)H3O+.
E)H2O.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
21
The pH of 0.10 M pyridine(aq)is 9.13.What is the value of Kb for pyridine?
A)2.7 104
B)7.4 1010
C)2.7 105
D)1.8 1010
E)1.8 109
A)2.7 104
B)7.4 1010
C)2.7 105
D)1.8 1010
E)1.8 109

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
22
The pH of 0.010 M aniline(aq)is 8.32.What is the percentage aniline protonated?
A)2.1%
B)0.69%
C)0.021%
D)0.12%
E)0.21%
A)2.1%
B)0.69%
C)0.021%
D)0.12%
E)0.21%

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
23
For a 0.10 M solution of a weak acid,HA,with pKa = 10,which of the following is true?
A)[HA] 0
B)[HA] = [A]
C)[HA] = [H3O+]
D)[HA] = Ka
E)[HA] [H3O+]
A)[HA] 0
B)[HA] = [A]
C)[HA] = [H3O+]
D)[HA] = Ka
E)[HA] [H3O+]

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
24
Write the charge balance equation for a dilute aqueous solution of HI.
A)[I] = [OH] + [H3O+]
B)[H3O+] = [OH]
C)[H3O+] = [I]
D)[H3O+] = [I] + [OH]
E)[HI]initial = [I]
A)[I] = [OH] + [H3O+]
B)[H3O+] = [OH]
C)[H3O+] = [I]
D)[H3O+] = [I] + [OH]
E)[HI]initial = [I]

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
25
When CaO(s)is dissolved in water,which of the following is true?
A)The solution contains O2(aq), OH(aq), and Ca2+(aq).
B)The solution contains CaO(aq).
C)CaO(s)does not dissolve in water.
D)The solution contains O2(aq)and Ca2+(aq).
E)The solution contains OH(aq)and Ca2+(aq).
A)The solution contains O2(aq), OH(aq), and Ca2+(aq).
B)The solution contains CaO(aq).
C)CaO(s)does not dissolve in water.
D)The solution contains O2(aq)and Ca2+(aq).
E)The solution contains OH(aq)and Ca2+(aq).

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following 0.10 M aqueous solutions gives the lowest pH?
A)CCl3COOH (pKa = 0.52)
B)Because all are acids, the pH is the same for all solutions.
C)HF (pKa = 3.45)
D)CH3COOH (pKa = 4.75)
E)H3PO4 (pKa1 = 2.12)
A)CCl3COOH (pKa = 0.52)
B)Because all are acids, the pH is the same for all solutions.
C)HF (pKa = 3.45)
D)CH3COOH (pKa = 4.75)
E)H3PO4 (pKa1 = 2.12)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
27
Estimate the pH of 107 M HClO4(aq).
A)6.8
B)8.0
C)1.0
D)5.0
E)7.0
A)6.8
B)8.0
C)1.0
D)5.0
E)7.0

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
28
What is the pH of 0.025 M (CH3)3N(aq)(Kb = 6.5 105)?
A)11.91
B)12.40
C)11.11
D)8.29
E)9.81
A)11.91
B)12.40
C)11.11
D)8.29
E)9.81

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is the strongest acid?
A)CH3CH2OH
B)CH3COOH
C)CHCl2COOH
D)CH2ClCOOH
E)CCl3COOH
A)CH3CH2OH
B)CH3COOH
C)CHCl2COOH
D)CH2ClCOOH
E)CCl3COOH

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
30
In liquid ammonia,the acid HB is a strong acid if it is a weaker proton donor than NH4+.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
31
The pH of 0.800 M aqueous benzenesulfonic acid is 0.51.What is the value of Ka for benzenesulfonic acid?
A)0.19
B)0.12
C)0.90
D)0.44
E)0.51
A)0.19
B)0.12
C)0.90
D)0.44
E)0.51

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
32
In liquid ammonia,the base B is a strong base if it is a stronger proton acceptor than NH2.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
33
The pH of 0.80 M benzenesulfonic acid is 0.51.What is the percentage ionization of benzenesulfonic acid?
A)25%
B)39%
C)51%
D)5.0%
E)64%
A)25%
B)39%
C)51%
D)5.0%
E)64%

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
34
Write the charge balance equation for a dilute aqueous solution of KOH.
A)[KOH]initial = [K+]
B)[OH] = [H3O+] + [K+]
C)[H3O+] = [OH]
D)[K+] = [OH] + [H3O+]
E)[OH] = [K+]
A)[KOH]initial = [K+]
B)[OH] = [H3O+] + [K+]
C)[H3O+] = [OH]
D)[K+] = [OH] + [H3O+]
E)[OH] = [K+]

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
35
A flask of 0.25 M HBrO(aq)has what pH? (pKa = 8.69)
A)5.90
B)0.60
C)8.10
D)4.65
E)9.30
A)5.90
B)0.60
C)8.10
D)4.65
E)9.30

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
36
For the following acids,which has the highest pKa?
A)HIO
B)HClO3
C)HClO
D)HBrO
E)HClO4
A)HIO
B)HClO3
C)HClO
D)HBrO
E)HClO4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
37
If the value of Kb for pyridine is 1.8 109,calculate the equilibrium constant for C5H5NH+(aq)+ H2O(l)↔ C5H5N(aq)+ H3O+(aq)
A)(1.8 109 )
B)1.8 1016
C)5.6 108
D)1.8 109
E)5.6 106
A)(1.8 109 )
B)1.8 1016
C)5.6 108
D)1.8 109
E)5.6 106

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
38
If the pKa of acetic acid is 4.75,the pKa of CH3CH2OH is
A)about 4.
B)much less than 4.75.
C)about 16.
D)also 4.75.
E)about 7.
A)about 4.
B)much less than 4.75.
C)about 16.
D)also 4.75.
E)about 7.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following 0.10 M aqueous solutions has the lowest pH?
A)B(OH)3
B)HIO
C)C2H5NH3Cl
D)C6H5OH
A)B(OH)3
B)HIO
C)C2H5NH3Cl
D)C6H5OH

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following aqueous solutions gives a pH greater than 7?
A)108 M NH4Cl
B)None of the solutions gives a pH greater than 7.
C)108 M CH3COOH
D)108 M HCl
E)108 M HCOOH
A)108 M NH4Cl
B)None of the solutions gives a pH greater than 7.
C)108 M CH3COOH
D)108 M HCl
E)108 M HCOOH

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
41
The Ka of phenol is 1.3 1010.For a solution labeled "1.0 103 M aqueous phenol,"
A)[H3O+] = [H3O+]2/[phenol]initial.
B)[H3O+] << [phenol]initial.
C)[H3O+] > 106.
D)pH ~ 4.
E)Kw/[H3O+] >> [phenol]initial.
A)[H3O+] = [H3O+]2/[phenol]initial.
B)[H3O+] << [phenol]initial.
C)[H3O+] > 106.
D)pH ~ 4.
E)Kw/[H3O+] >> [phenol]initial.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
42
For a solution labeled "0.10 M H3PO4(aq),"
A)[H2PO4] is greater than 0.10 M.
B)[H+] = 0.30 M.
C)[PO43] = 0.10 M.
D)[H+] = 0.10 M.
E)[H+] is less than 0.10 M.
A)[H2PO4] is greater than 0.10 M.
B)[H+] = 0.30 M.
C)[PO43] = 0.10 M.
D)[H+] = 0.10 M.
E)[H+] is less than 0.10 M.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
43
The following 0.1 M aqueous solutions are arranged in order of increasing pH,with the highest pH on the far right. 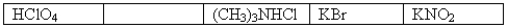 Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
A)NaI
B)HCOOH
C)C6H5NH2
D)CH3NH3Cl
E)NaClO
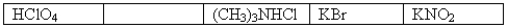 Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
Which one of the following 0.10 M aqueous solutions should be placed in the empty box?A)NaI
B)HCOOH
C)C6H5NH2
D)CH3NH3Cl
E)NaClO

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
44
For a solution labeled "0.10 M H2SO4(aq),"
A)[HSO4] is greater than 0.10 M.
B)the pH is less than 1.0.
C)[SO42] = 0.10 M.
D)the pH equals 1.0.
E)the pH is greater than 1.0.
A)[HSO4] is greater than 0.10 M.
B)the pH is less than 1.0.
C)[SO42] = 0.10 M.
D)the pH equals 1.0.
E)the pH is greater than 1.0.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
45
The equation that represents Ka2 for phosphoric acid is
A)HPO42(aq)+ H2O(l)↔ PO43(aq)+ H3O+(aq)
B)H2PO4(aq)+ H2O(l)↔ HPO42(aq)+ H3O+(aq).
C)H3PO4(aq)+ 2H2O(l)↔ HPO42(aq)+ 2H3O+(aq).
D)HPO42(aq)+ H2O(l)↔ H2PO4(aq)+ OH(aq).
E)H3PO4(aq)+ H2O(l)↔ H2PO4(aq)+ H3O+(aq).
A)HPO42(aq)+ H2O(l)↔ PO43(aq)+ H3O+(aq)
B)H2PO4(aq)+ H2O(l)↔ HPO42(aq)+ H3O+(aq).
C)H3PO4(aq)+ 2H2O(l)↔ HPO42(aq)+ 2H3O+(aq).
D)HPO42(aq)+ H2O(l)↔ H2PO4(aq)+ OH(aq).
E)H3PO4(aq)+ H2O(l)↔ H2PO4(aq)+ H3O+(aq).

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
46
The following 0.1 M aqueous solutions are arranged in order of increasing pH,with the highest pH on the far right.  Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
A)CuSO4
B)NaNO2
C)CH3NH2
D)NaHCO3
E)Na2HPO4
 Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
Which one of the following 0.10 M aqueous solutions should be placed in the empty box?A)CuSO4
B)NaNO2
C)CH3NH2
D)NaHCO3
E)Na2HPO4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
47
What is the [H+] for a solution labeled "0.0500 M H2SO3(aq)" if pKa1 = 1.81,and pKa2 = 6.91?
A)0.021 M
B)0.029 M
C)0.015 M
D)0.025 M
E)0.050 M
A)0.021 M
B)0.029 M
C)0.015 M
D)0.025 M
E)0.050 M

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
48
Calculate the equilibrium concentration of sulfurous acid in a solution labeled "0.100 M H2SO3(aq)" if pKa1 = 1.81,and pKa2 = 6.91.
A)0.068 M
B)0.015 M
C)0.100 M
D)0.050 M
E)0.032 M
A)0.068 M
B)0.015 M
C)0.100 M
D)0.050 M
E)0.032 M

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
49
Calculate the equilibrium constant for the reaction HS(aq)+ H2O(l)↔ H2S(aq)+ OH(aq),
Given Ka1 = 1.3 107 and Ka2 = 7.1 1015 for H2S.
A)1.3 107
B)7.7 108
C)9.2 1022
D)7.1 1015
E)1.4
Given Ka1 = 1.3 107 and Ka2 = 7.1 1015 for H2S.
A)1.3 107
B)7.7 108
C)9.2 1022
D)7.1 1015
E)1.4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
50
A 0.0010 M solution of a weak acid,HA,with Ka = 2 1010 produces [H3O+] < 106 M.Which of the following equations can be used to determine [H3O+]?
A)The acid is so weak that the pH is about 7.
B)[H3O+]2 + Ka [H3O+] [HA]initialKa = 0
C)[H3O+] = (Kw + Ka[HA]initial)½
D)[H3O+] = [HA]initial
E)[H3O+] = (Ka[HA]initial)½
A)The acid is so weak that the pH is about 7.
B)[H3O+]2 + Ka [H3O+] [HA]initialKa = 0
C)[H3O+] = (Kw + Ka[HA]initial)½
D)[H3O+] = [HA]initial
E)[H3O+] = (Ka[HA]initial)½

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
51
The following 0.1 M aqueous solutions are arranged in order of increasing pH,with the highest pH on the far right.  Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
A)NaHSO4
B)KF
C)HNO2
D)CH3NH2
E)(CH3)3NHCl
 Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
Which one of the following 0.10 M aqueous solutions should be placed in the empty box?A)NaHSO4
B)KF
C)HNO2
D)CH3NH2
E)(CH3)3NHCl

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
52
The equation that represents Ka2 for sulfurous acid is
A)HSO3(aq)+ H2O(l)↔ H2SO3(aq)+ OH(aq).
B)HSO3(aq)+ H2O(l)↔ SO32(aq)+ H3O+(aq).
C)H2SO3(aq)+ 2H2O(l)↔ SO32(aq)+ 2H3O+(aq).
D)SO32(aq)+ H2O(l)↔ HSO3(aq)+ OH(aq).
E)H2SO3(aq)+ H2O(l)↔ HSO3(aq)+ H3O+(aq).
A)HSO3(aq)+ H2O(l)↔ H2SO3(aq)+ OH(aq).
B)HSO3(aq)+ H2O(l)↔ SO32(aq)+ H3O+(aq).
C)H2SO3(aq)+ 2H2O(l)↔ SO32(aq)+ 2H3O+(aq).
D)SO32(aq)+ H2O(l)↔ HSO3(aq)+ OH(aq).
E)H2SO3(aq)+ H2O(l)↔ HSO3(aq)+ H3O+(aq).

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
53
For a solution labeled "0.10 M H2SO3(aq)," pKa1 = 1.81 and pKa2 = 6.91,which of the following is true?
A)[H+] = 0.2 M.
B)The pH is 1.0.
C)The pH ~1.5.
D)The pH is 0.70.
E)The pH ~ 4.4.
A)[H+] = 0.2 M.
B)The pH is 1.0.
C)The pH ~1.5.
D)The pH is 0.70.
E)The pH ~ 4.4.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
54
The following 0.1 M aqueous solutions are arranged in order of increasing pH,with the highest pH on the far right.  Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
A)NH4Cl
B)NaCN
C)Al2(SO4)3
D)CH3NH2
E)CH3COOH
 Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
Which one of the following 0.10 M aqueous solutions should be placed in the empty box?A)NH4Cl
B)NaCN
C)Al2(SO4)3
D)CH3NH2
E)CH3COOH

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
55
The following 0.1 M aqueous solutions are arranged in order of increasing pH,with the highest pH on the far right.  Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
A)NaCN
B)CH3COOH
C)KNO2
D)NaBr
E)NaHSO4
 Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
Which one of the following 0.10 M aqueous solutions should be placed in the empty box?A)NaCN
B)CH3COOH
C)KNO2
D)NaBr
E)NaHSO4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
56
The Ka of phenol is 1.3 1010.For a solution labeled "1.0 103 M aqueous phenol,"
A)Kw/[H3O+] >> [phenol]initial.
B)Kw/[H3O+] << [phenol]initial.
C)[H3O+] > 106.
D)pH ~ 4.
E)[H3O+] = [H3O+]2/[phenol]initial.
A)Kw/[H3O+] >> [phenol]initial.
B)Kw/[H3O+] << [phenol]initial.
C)[H3O+] > 106.
D)pH ~ 4.
E)[H3O+] = [H3O+]2/[phenol]initial.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
57
Estimate the pH of 107 M KOH(aq).
A)6.9
B)9
C)13
D)7.2
E)7.0
A)6.9
B)9
C)13
D)7.2
E)7.0

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
58
Write the charge balance equation for a solution that is 0.0010 M phenol(aq).Let phenol be represented by HA(aq).
A)[H3O+] = [OH]
B)Kw = [H3O+][OH]
C)[H3O+] = [OH] + [A]
D)Ka = Kw/Kb
E)0.0010 = [HA] + [A]
A)[H3O+] = [OH]
B)Kw = [H3O+][OH]
C)[H3O+] = [OH] + [A]
D)Ka = Kw/Kb
E)0.0010 = [HA] + [A]

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
59
If pKa1 and pKa2 for H2S are 6.88 and 14.15,respectively,calculate the equilibrium constant for the reaction below: H2S(aq)+ 2H2O(l)↔ 2H3O+(aq)+ S2(aq)
A)1.3 107
B)1.1 107
C)7.7 108
D)9.2 1022
E)7.1 1015
A)1.3 107
B)1.1 107
C)7.7 108
D)9.2 1022
E)7.1 1015

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
60
If pKa1 and pKa2 for H2CO3 are 6.37 and 10.25,respectively,calculate the equilibrium constant for the reaction below: H2CO3(aq)+ 2H2O(l)↔ 2H3O+(aq)+ CO32(aq)
A)4.1 1011
B)4.3 107
C)5.6 1011
D)2.3 108
E)2.4 1017
A)4.1 1011
B)4.3 107
C)5.6 1011
D)2.3 108
E)2.4 1017

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
61
Calculate the equilibrium constant for the reaction S2(aq)+ H2O(l)↔ HS(aq)+ OH(aq),
Given Ka1 = 1.3 107 and Ka2 = 7.1 1015 for H2S.
A)1.3 107
B)9.2 1022
C)7.7 108
D)7.1 1015
E)1.4
Given Ka1 = 1.3 107 and Ka2 = 7.1 1015 for H2S.
A)1.3 107
B)9.2 1022
C)7.7 108
D)7.1 1015
E)1.4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
62
Both H2O and OH can act as a Brønsted acid and a Brønsted base but not as a Lewis acid.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
63
In a solution labeled "0.0018 M barium hydroxide" what is the molarity of OH?
A)0.0018 M
B)0.00090 M
C)0.0036 M
D)0.0072 M
E)None of the above.
A)0.0018 M
B)0.00090 M
C)0.0036 M
D)0.0072 M
E)None of the above.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
64
If (HSO3)= 0.45 at pH 7.0,what are (H2SO3)and (SO32)at this pH? For H2SO3,pKa1 and pKa2 are 1.81 and 6.91,respectively.
A)((H2SO3)= 0.225 and (SO32)= 0.55)
B)((H2SO3)= 0.55 and (SO32)~ 0)
C)((H2SO3)~ 0 and (SO32)= 0.225)
D)((H2SO3)~ 0 and (SO32)= 0.55)
E)((H2SO3)= 0.45 and (SO32)= 0.55)
A)((H2SO3)= 0.225 and (SO32)= 0.55)
B)((H2SO3)= 0.55 and (SO32)~ 0)
C)((H2SO3)~ 0 and (SO32)= 0.225)
D)((H2SO3)~ 0 and (SO32)= 0.55)
E)((H2SO3)= 0.45 and (SO32)= 0.55)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
65
The pH of 0.010 M H3PO4(aq)is 2.24.Estimate the concentration of HPO42 in the solution.For H3PO4,the values of Ka1,Ka2,and Ka3 are 7.6 103,6.2 108,and 2.1 1013,respectively.
A)5.8 103 M
B)7.6 103 M
C)0.010 M
D)6.2 108 M
E)2.1 1013 M
A)5.8 103 M
B)7.6 103 M
C)0.010 M
D)6.2 108 M
E)2.1 1013 M

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
66
The amino acid methionine,HOOC-CH(CH2CH2SCH3)NH3+,has pKa1 = 2.2 and pKa2 = 9.1.If this amino acid is represented by H2L+,the major species at pH 6 is
A)HL
B)H2L+
C)L
D)HL and L
A)HL
B)H2L+
C)L
D)HL and L

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
67
The amino acid alanine,HOOC-CH(CH3)NH3+,has Ka1 = 4.5 103 and Ka2 = 1.4 1010.Calculate (OOC-CH(CH3)NH3+)at pH 3.
A)0
B)0.82
C)0.18
D)0.29
E)0.58
A)0
B)0.82
C)0.18
D)0.29
E)0.58

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
68
All of the following acids have the same strength in water except
A)HNO3
B)HClO3
C)HBr
D)HF
A)HNO3
B)HClO3
C)HBr
D)HF

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
69
The fractional composition diagram for the amino acid alanine is given below. 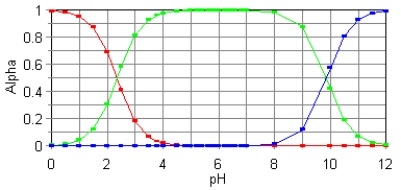 Write the structure of the dominant species at pH 1,6,and 12,respectively.
Write the structure of the dominant species at pH 1,6,and 12,respectively.
 Write the structure of the dominant species at pH 1,6,and 12,respectively.
Write the structure of the dominant species at pH 1,6,and 12,respectively.
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
70
The amino acid alanine,HOOC-CH(CH3)NH3+,has Ka1 = 4.5 103 and Ka2 = 1.4 1010.Calculate (HOOC-CH(CH3)NH3+)at pH 3.
A)0
B)0.82
C)0.58
D)0.29
E)0.18
A)0
B)0.82
C)0.58
D)0.29
E)0.18

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
71
The boxes below contain a series of 0.1 M aqueous solutions of increasing pH where A is the solution of lowest pH and E is the solution of highest pH.  Match each box with the correct compound.
Match each box with the correct compound.
phenol,pKa = 9.89
cyanide ion,pKb = 4.69
pyridine,pKb = 8.75
hydrogen sulfate ion,pKa = 1.92
sodium nitrate
 Match each box with the correct compound.
Match each box with the correct compound.phenol,pKa = 9.89
cyanide ion,pKb = 4.69
pyridine,pKb = 8.75
hydrogen sulfate ion,pKa = 1.92
sodium nitrate

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
72
The pH of 0.010 M H3PO4(aq)is 2.24; estimate the concentration of PO43 in the solution.For H3PO4,the values of Ka1,Ka2,and Ka3 are 7.6 103,6.2 108,and 2.1 1013,respectively.
A)5.8 103 M
B)2.1 1013 M
C)7.6 103 M
D)6.2 108 M
E)2.3 1018 M
A)5.8 103 M
B)2.1 1013 M
C)7.6 103 M
D)6.2 108 M
E)2.3 1018 M

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
73
The amino acid alanine,HOOC-CH(CH3)NH3+,has Ka1 = 4.5 103 and Ka2 = 1.4 1010.Calculate (OOC-CH(CH3)NH3+)at pH 10.
A)0.42
B)0.29
C)1.0
D)0
E)0.58
A)0.42
B)0.29
C)1.0
D)0
E)0.58

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
74
All of the following are Lewis bases except
A)OH
B)H2O
C)SO3
D)Br
A)OH
B)H2O
C)SO3
D)Br

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
75
If (HSO3)= 0.83 at pH 2.5,what are (H2SO3)and (SO32)at this pH? For H2SO3,pKa1 and pKa2 are 1.81 and 6.91,respectively.
A)((H2SO3)~ 0 and (SO32)= 0.17)
B)((H2SO3)= 0.415 and (SO32)~ 0)
C)((H2SO3)= 0.0.085 and (SO32)= 0.085)
D)((H2SO3)= 0.17 and (SO32)~ 0)
E)((H2SO3)= 0.17 and (SO32)~ 1)
A)((H2SO3)~ 0 and (SO32)= 0.17)
B)((H2SO3)= 0.415 and (SO32)~ 0)
C)((H2SO3)= 0.0.085 and (SO32)= 0.085)
D)((H2SO3)= 0.17 and (SO32)~ 0)
E)((H2SO3)= 0.17 and (SO32)~ 1)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
76
True or false: the pH of 0.10 M and 0.40 M NaHCO3(aq)solutions is 8.31 for both?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
77
Estimate the pH of 0.10 M Na2HPO4(aq)given pKa1 = 2.12,pKa2 = 7.21,and pKa3 = 12.68 for phosphoric acid.
A)12.68
B)9.94
C)7.40
D)4.67
E)2.12
A)12.68
B)9.94
C)7.40
D)4.67
E)2.12

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
78
Of the following,which is not a Lewis acid?
A)H3O+
B)SO3
C)NO2
D)BF3
E)None of the above.
A)H3O+
B)SO3
C)NO2
D)BF3
E)None of the above.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
79
The fractional composition diagram for the amino acid alanine is shown below. 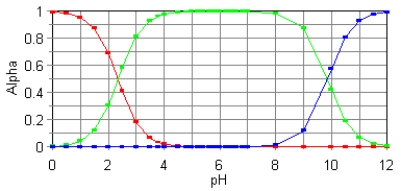 What do the two points represent where alpha is 0.5?
What do the two points represent where alpha is 0.5?
 What do the two points represent where alpha is 0.5?
What do the two points represent where alpha is 0.5?
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
80
For a solution of phosphoric acid,write the equation for (HPO42).
A)Ka3/([H3O+]3 + Ka1[H3O+]2 + Ka1Ka2[H3O+] + Ka1Ka2Ka3)
B)[H3O+]/([H3O+]3 + Ka1[H3O+]2 + Ka1Ka2[H3O+] + Ka1Ka2Ka3)
C)Ka1Ka2[H3O+]/([H3O+]3 + Ka1[H3O+]2 + Ka1Ka2[H3O+] + Ka1Ka2Ka3)
D)Ka1Ka2Ka3/([H3O+]3 + Ka1[H3O+]2 + Ka1Ka2[H3O+] + Ka1Ka2Ka3)
E)Ka1[H3O+]2/([H3O+]3 + Ka1[H3O+]2 + Ka1Ka2[H3O+] + Ka1Ka2Ka3)
A)Ka3/([H3O+]3 + Ka1[H3O+]2 + Ka1Ka2[H3O+] + Ka1Ka2Ka3)
B)[H3O+]/([H3O+]3 + Ka1[H3O+]2 + Ka1Ka2[H3O+] + Ka1Ka2Ka3)
C)Ka1Ka2[H3O+]/([H3O+]3 + Ka1[H3O+]2 + Ka1Ka2[H3O+] + Ka1Ka2Ka3)
D)Ka1Ka2Ka3/([H3O+]3 + Ka1[H3O+]2 + Ka1Ka2[H3O+] + Ka1Ka2Ka3)
E)Ka1[H3O+]2/([H3O+]3 + Ka1[H3O+]2 + Ka1Ka2[H3O+] + Ka1Ka2Ka3)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck


