Exam 12: Acids and Bases
Exam 1: The Quantum World99 Questions
Exam 2: Quantum Mechanics in Action: Atoms100 Questions
Exam 3: Chemical Bonds84 Questions
Exam 4: Molecular Shape and Structure95 Questions
Exam 5: The Properties of Gases94 Questions
Exam 6: Liquids and Solids94 Questions
Exam 7: Inorganic Materials99 Questions
Exam 8: Thermodynamics: the First Law94 Questions
Exam 9: Thermodynamics: the Second and Third Laws93 Questions
Exam 10: Physical Equilibria94 Questions
Exam 11: Chemical Equilibria93 Questions
Exam 12: Acids and Bases94 Questions
Exam 13: Aqueous Equilibria94 Questions
Exam 14: Electrochemistry94 Questions
Exam 15: Chemical Kinetics88 Questions
Exam 16: The Elements: the Main Group Elements186 Questions
Exam 17: The Elements: the D Block93 Questions
Exam 18: Nuclear Chemistry95 Questions
Exam 19: Organic Chemistry I93 Questions
Exam 20: Organic Chemistry II94 Questions
Select questions type
All of the following 0.1 M aqueous solutions are acidic except
Free
(Multiple Choice)
4.7/5  (37)
(37)
Correct Answer:
A
The following 0.1 M aqueous solutions are arranged in order of increasing pH,with the highest pH on the far right.  Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
A
Which of the following is the strongest acid?
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
E
The boxes below contain a series of 0.1 M aqueous solutions of increasing pH where A is the solution of lowest pH and E is the solution of highest pH.  Match each box with the correct compound.
phenol,pKa = 9.89
cyanide ion,pKb = 4.69
pyridine,pKb = 8.75
hydrogen sulfate ion,pKa = 1.92
sodium nitrate
Match each box with the correct compound.
phenol,pKa = 9.89
cyanide ion,pKb = 4.69
pyridine,pKb = 8.75
hydrogen sulfate ion,pKa = 1.92
sodium nitrate
(Short Answer)
4.8/5  (31)
(31)
In liquid ammonia,the acid HB is a strong acid if it is a weaker proton donor than NH4+.True or false?
(True/False)
4.8/5  (37)
(37)
The following 0.1 M aqueous solutions are arranged in order of increasing pH,with the highest pH on the far right. 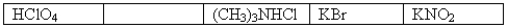 Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
(Multiple Choice)
4.8/5  (32)
(32)
Calculate the equilibrium constant for the reaction S2(aq)+ H2O(l)↔ HS(aq)+ OH(aq),
Given Ka1 = 1.3 107 and Ka2 = 7.1 1015 for H2S.
(Multiple Choice)
4.9/5  (35)
(35)
All of the following acids have the same strength in water except
(Multiple Choice)
4.8/5  (48)
(48)
The amino acid methionine,HOOC-CH(CH2CH2SCH3)NH3+,has pKa1 = 2.2 and pKa2 = 9.1.If this amino acid is represented by H2L+,the major species at pH 6 is
(Multiple Choice)
4.8/5  (42)
(42)
The following 0.1 M aqueous solutions are arranged in order of increasing pH,with the highest pH on the far right.  Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
(Multiple Choice)
4.8/5  (29)
(29)
Which of the following aqueous solutions gives a pH greater than 7?
(Multiple Choice)
4.7/5  (42)
(42)
What is the molarity of OH in a 0.0018 M calcium hydroxide solution?
(Multiple Choice)
4.9/5  (28)
(28)
If (HSO3)= 0.45 at pH 7.0,what are (H2SO3)and (SO32)at this pH? For H2SO3,pKa1 and pKa2 are 1.81 and 6.91,respectively.
(Multiple Choice)
4.8/5  (44)
(44)
For a 0.10 M solution of a weak acid,HA,with pKa = 10,which of the following is true?
(Multiple Choice)
4.8/5  (34)
(34)
HBrO(aq),in a 0.25 M solution,has a pKa = 8.69.What is its pH?
(Multiple Choice)
5.0/5  (29)
(29)
Showing 1 - 20 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)