Deck 20: Electrochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/113
Play
Full screen (f)
Deck 20: Electrochemistry
1
What is the coefficient of 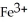 when the following equation is balanced?
when the following equation is balanced?
CN- + Fe3+ → CNO- + Fe2+ (basic solution)
A)1
B)2
C)3
D)4
E)5
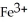 when the following equation is balanced?
when the following equation is balanced?CN- + Fe3+ → CNO- + Fe2+ (basic solution)
A)1
B)2
C)3
D)4
E)5
2
2
Which one of the following reactions is a redox reaction?
A)NaOH + HCl → NaCl + H2O
B)Pb2+ + 2Cl- → PbCl2
C)AgNO3 + HCl → HNO3 + AgCl
D)None of the above is a redox reaction.
A)NaOH + HCl → NaCl + H2O
B)Pb2+ + 2Cl- → PbCl2
C)AgNO3 + HCl → HNO3 + AgCl
D)None of the above is a redox reaction.
None of the above is a redox reaction.
3
Which of the following reactions will occur spontaneously as written?
A)3Fe2+ (aq)+ Cr3+ (aq)→ Cr (s)+ 3Fe3+ (aq)
B)2Cr3+ (aq)+ 3Sn2+ (aq)→ 3Sn4+ (aq)+ 2Cr (s)
C)Sn4+ (aq)+ Fe2+ (s)→ Sn2+ (aq)+ Fe (s)
D)Sn2+ (aq)+ Fe2+ (s)→ Sn4+ (aq)+ Fe3+ (aq)
E)2Cr (s)+ 3Fe2+ (s)→ 3Fe (s)+ 2Cr3+ (aq)
A)3Fe2+ (aq)+ Cr3+ (aq)→ Cr (s)+ 3Fe3+ (aq)
B)2Cr3+ (aq)+ 3Sn2+ (aq)→ 3Sn4+ (aq)+ 2Cr (s)
C)Sn4+ (aq)+ Fe2+ (s)→ Sn2+ (aq)+ Fe (s)
D)Sn2+ (aq)+ Fe2+ (s)→ Sn4+ (aq)+ Fe3+ (aq)
E)2Cr (s)+ 3Fe2+ (s)→ 3Fe (s)+ 2Cr3+ (aq)
2Cr (s)+ 3Fe2+ (s)→ 3Fe (s)+ 2Cr3+ (aq)
4
Which of the following reactions will occur spontaneously as written?
A)Sn4+ (aq)+ Fe3+ (aq)→ Sn2+ (aq)+ Fe2+ (aq)
B)3Fe (s)+ 2Cr3+ (aq)→ 2Cr (s)+ 3Fe2+ (aq)
C)Sn4+ (aq)+ Fe2+ (aq)→ Sn2+ (aq)+ Fe (s)
D)3Sn4+ (aq)+ 2Cr (s)→ 2Cr3+ (aq)+ 3Sn2+ (aq)
E)3Fe2+ (aq)→ Fe (s)+ 2Fe3+ (aq)
A)Sn4+ (aq)+ Fe3+ (aq)→ Sn2+ (aq)+ Fe2+ (aq)
B)3Fe (s)+ 2Cr3+ (aq)→ 2Cr (s)+ 3Fe2+ (aq)
C)Sn4+ (aq)+ Fe2+ (aq)→ Sn2+ (aq)+ Fe (s)
D)3Sn4+ (aq)+ 2Cr (s)→ 2Cr3+ (aq)+ 3Sn2+ (aq)
E)3Fe2+ (aq)→ Fe (s)+ 2Fe3+ (aq)

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the halogens in Table 20.1 is the strongest oxidizing agent?
A)Cl2
B)Br2
C)F2
D)I2
E)All of the halogens have equal strength as oxidizing agents.
A)Cl2
B)Br2
C)F2
D)I2
E)All of the halogens have equal strength as oxidizing agents.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
6
Which transformation could take place at the anode of an electrochemical cell?
A)Cr2O72- → Cr2+
B)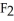 to
to 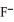
C)O2 to H2O
D)HAsO2 to As
E)None of the above could take place at the anode.
A)Cr2O72- → Cr2+
B)
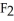 to
to 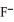
C)O2 to H2O
D)HAsO2 to As
E)None of the above could take place at the anode.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
7
Which element is oxidized in the reaction below?
Fe(CO)5 (l)+ 2HI (g)→ Fe(CO)4I2 (s)+ CO (g)+ H2 (g)
A)C
B)O
C)H
D)Fe
E)I
Fe(CO)5 (l)+ 2HI (g)→ Fe(CO)4I2 (s)+ CO (g)+ H2 (g)
A)C
B)O
C)H
D)Fe
E)I

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
8
Consider an electrochemical cell based on the reaction: 2H+ (aq)+ Sn (s)→ Sn2+ (aq)+ H2 (g)
Which of the following actions would change the measured cell potential?
A)increasing the pH in the cathode compartment
B)lowering the pH in the cathode compartment
C)increasing the [Sn2+] in the anode compartment
D)increasing the pressure of hydrogen gas in the cathode compartment
E)Any of the above will change the measure cell potential.
Which of the following actions would change the measured cell potential?
A)increasing the pH in the cathode compartment
B)lowering the pH in the cathode compartment
C)increasing the [Sn2+] in the anode compartment
D)increasing the pressure of hydrogen gas in the cathode compartment
E)Any of the above will change the measure cell potential.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
9
The purpose of the salt bridge in an electrochemical cell is to ________.
A)maintain electrical neutrality in the half-cells via migration of ions
B)provide a source of ions to react at the anode and cathode
C)provide oxygen to facilitate oxidation at the anode
D)provide a means for electrons to travel from the anode to the cathode
E)provide a means for electrons to travel from the cathode to the anode
A)maintain electrical neutrality in the half-cells via migration of ions
B)provide a source of ions to react at the anode and cathode
C)provide oxygen to facilitate oxidation at the anode
D)provide a means for electrons to travel from the anode to the cathode
E)provide a means for electrons to travel from the cathode to the anode

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following reactions is a redox reaction?
(a)K2CrO4 + BaCl2 → BaCrO4 + 2KCl
(b)Pb22+ + 2Br- → PbBr
(c)Cu + S → CuS
A)(a)only
B)(b)only
C)(c)only
D)(a)and (c)
E)(b)and (c)
(a)K2CrO4 + BaCl2 → BaCrO4 + 2KCl
(b)Pb22+ + 2Br- → PbBr
(c)Cu + S → CuS
A)(a)only
B)(b)only
C)(c)only
D)(a)and (c)
E)(b)and (c)

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
11
Which one of the following types of elements is most likely to be a good oxidizing agent?
A)alkali metals
B)lanthanides
C)alkaline earth elements
D)transition elements
E)halogens
A)alkali metals
B)lanthanides
C)alkaline earth elements
D)transition elements
E)halogens

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
12
Which one of the following is the best oxidizing agent?
A)H2
B)Na
C)O2
D)Li
E)Ca
A)H2
B)Na
C)O2
D)Li
E)Ca

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
13
Which transformation could take place at the cathode of an electrochemical cell?
A)MnO2 → MnO4-
B)Br2 → BrO3-
C)NO → HNO2
D)HSO4- → H2SO3
E)Mn2+ → MnO4-
A)MnO2 → MnO4-
B)Br2 → BrO3-
C)NO → HNO2
D)HSO4- → H2SO3
E)Mn2+ → MnO4-

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
14
Which element is oxidized in the reaction below?
I- + MnO4- + H+ → I2 + MnO2 + H2O
A)H
B)I
C)O
D)Mn
I- + MnO4- + H+ → I2 + MnO2 + H2O
A)H
B)I
C)O
D)Mn

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
15
Which transformation below is an example of an oxidation in an electrochemical cell?
A)CO2 → C2O42-
B)VO2+ → VO2+
C)NO → NO3-
D)H2AsO4 → H3AsO3
E)O2 → H2O2
A)CO2 → C2O42-
B)VO2+ → VO2+
C)NO → NO3-
D)H2AsO4 → H3AsO3
E)O2 → H2O2

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
16
What is the coefficient of the dichromate ion when the following equation is balanced?
Fe2+ + Cr2O72- → Fe3+ + Cr3+ (acidic solution)
A)1
B)2
C)3
D)5
E)6
Fe2+ + Cr2O72- → Fe3+ + Cr3+ (acidic solution)
A)1
B)2
C)3
D)5
E)6

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
17
Consider an electrochemical cell based on the reaction: 2H+ (aq)+ Sn (s)→ Sn2+ (aq)+ H2 (g)
Which of the following actions would not change the measured cell potential?
A)lowering the pH in the cathode compartment
B)addition of more tin metal to the anode compartment
C)increasing the tin (II)ion concentration in the anode compartment
D)increasing the pressure of hydrogen gas in the cathode compartment
E)Any of the above will change the measured cell potential.
Which of the following actions would not change the measured cell potential?
A)lowering the pH in the cathode compartment
B)addition of more tin metal to the anode compartment
C)increasing the tin (II)ion concentration in the anode compartment
D)increasing the pressure of hydrogen gas in the cathode compartment
E)Any of the above will change the measured cell potential.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
18
What is the coefficient of the permanganate ion when the following equation is balanced?
MnO4- + Br- → Mn2+ + Br2 (acidic solution)
A)1
B)2
C)3
D)5
E)4
MnO4- + Br- → Mn2+ + Br2 (acidic solution)
A)1
B)2
C)3
D)5
E)4

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
19
Which substance is the oxidizing agent in the following reaction?
Fe2S3 + 12HNO3 → 2Fe(NO3)3 + 3S + 6NO2 + 6H2O
A)HNO3
B)S
C)NO2
D)Fe2S3
E)H2O
Fe2S3 + 12HNO3 → 2Fe(NO3)3 + 3S + 6NO2 + 6H2O
A)HNO3
B)S
C)NO2
D)Fe2S3
E)H2O

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
20
Which element is oxidized in the reaction below?
Fe2+ + H+ + Cr2O72- → Fe3+ + Cr3+ + H2O
A)Cr
B)O
C)H
D)Fe
Fe2+ + H+ + Cr2O72- → Fe3+ + Cr3+ + H2O
A)Cr
B)O
C)H
D)Fe

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
21
________ is the reducing agent in the reaction below. Cr2O72- + 6S2O32- + 14H+ → 2Cr3+ + 3S4O62- + 7H2O
A)Cr2O72-
B)S2O32-
C)H+
D)Cr3+
E)S4O62-
A)Cr2O72-
B)S2O32-
C)H+
D)Cr3+
E)S4O62-

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
22
What is the oxidation number of oxygen in K2O2?
A)-2
B)+1
C)-1
D)+2
E)-1/2
A)-2
B)+1
C)-1
D)+2
E)-1/2

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
23
Which substance is the oxidizing agent in the reaction below?
Pb + PbO2 + 2H2SO4 → 2PbSO4 + 2H2O
A)Pb
B)PbSO4
C)H2SO4
D)PbO2
E)H2O
Pb + PbO2 + 2H2SO4 → 2PbSO4 + 2H2O
A)Pb
B)PbSO4
C)H2SO4
D)PbO2
E)H2O

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
24
Which element is oxidized in the following reaction?
Cr2O72- + 6S2O32- + 14H+ → 2Cr3+ + 3S4O62- + 7H2O
A)S4O62-
B)Cr
C)H
D)O
E)S
Cr2O72- + 6S2O32- + 14H+ → 2Cr3+ + 3S4O62- + 7H2O
A)S4O62-
B)Cr
C)H
D)O
E)S

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
25
The loss of electrons by an element is called ________.
A)fractionation
B)reduction
C)disproportionation
D)oxidation
E)sublimation
A)fractionation
B)reduction
C)disproportionation
D)oxidation
E)sublimation

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
26
________ is the reducing agent in the reaction below. Na(s)+ 2Cl2(g)→ 2NaCl(s)
A)Cl2
B)NaCl
C)Na+
D)Na
E)Cl-
A)Cl2
B)NaCl
C)Na+
D)Na
E)Cl-

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
27
________ is the oxidizing agent in the reaction below. Na(s)+ 2Cl2(g)→ 2NaCl(s)
A)Na+
B)Cl-
C)Na
D)NaCl
E)Cl2
A)Na+
B)Cl-
C)Na
D)NaCl
E)Cl2

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
28
In a lead-acid battery,the electrodes are consumed.In this battery,________.
A)the anode is Pb
B)the anode is PbSO4
C)the anode is PbO2
D)the cathode is PbSO4
E)the cathode is Pb
A)the anode is Pb
B)the anode is PbSO4
C)the anode is PbO2
D)the cathode is PbSO4
E)the cathode is Pb

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
29
Which substance is serving as the oxidizing agent in the following reaction?
CH4(g)+ 2O2(g)→ CO2(g)+ 2H2O(g)
A)H2O
B)CH4
C)O2
D)CO2
E)The reaction above is not a redox reaction
CH4(g)+ 2O2(g)→ CO2(g)+ 2H2O(g)
A)H2O
B)CH4
C)O2
D)CO2
E)The reaction above is not a redox reaction

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
30
What is being reduced at the cathode in the hydrogen fuel cell?
A)O2
B)KOH
C)Li
D)H2
E)Pt
A)O2
B)KOH
C)Li
D)H2
E)Pt

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
31
Which element is reduced in the following reaction?
Cr2O72- + 6S2O32- + 14H+ → 2Cr3+ + 3S4O62- + 7H2O
A)H
B)Cr
C)O
D)S
E)S4O62-
Cr2O72- + 6S2O32- + 14H+ → 2Cr3+ + 3S4O62- + 7H2O
A)H
B)Cr
C)O
D)S
E)S4O62-

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
32
What is the oxidation number of manganese in MnO2?
A)+3
B)+2
C)+1
D)+4
E)+7
A)+3
B)+2
C)+1
D)+4
E)+7

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
33
What is the cathode in an alkaline battery?
A)MnO2
B)KOH
C)Zn powder
D)Mn2O3
E)Pt
A)MnO2
B)KOH
C)Zn powder
D)Mn2O3
E)Pt

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
34
One of the differences between a voltaic cell and an electrolytic cell is that in an electrolytic cell,________.
A)an electric current is produced by a chemical reaction
B)electrons flow toward the anode
C)a nonspontaneous reaction is forced to occur
D)O2 gas is produced at the cathode
E)oxidation occurs at the cathode
A)an electric current is produced by a chemical reaction
B)electrons flow toward the anode
C)a nonspontaneous reaction is forced to occur
D)O2 gas is produced at the cathode
E)oxidation occurs at the cathode

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
35
What is the oxidation number of oxygen in H2O2?
A)0
B)+1
C)+2
D)-1
E)-2
A)0
B)+1
C)+2
D)-1
E)-2

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
36
What is the oxidation number of manganese in KMnO4?
A)+1
B)+2
C)+7
D)+4
E)+5
A)+1
B)+2
C)+7
D)+4
E)+5

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the statements about cathodic protection of a metal pipe against corrosion is correct?
A)The metal pipe is protected by attaching an active metal to make the pipe the cathode in an electrochemical cell
B)The metal pipe is protected by coating the pipe with another metal whose standard reduction potential is less negative than that of the pipe
C)The metal pipe is protected by attaching a dry cell to reduce any metal ions which might be formed
D)The metal pipe is protected by attaching an active metal to make the pipe the anode in an electrochemical cell
E)The metal pipe is protected by coating the pipe with a fluoropolymer to act as a source of fluoride ion (since the latter is so hard to oxidize)
A)The metal pipe is protected by attaching an active metal to make the pipe the cathode in an electrochemical cell
B)The metal pipe is protected by coating the pipe with another metal whose standard reduction potential is less negative than that of the pipe
C)The metal pipe is protected by attaching a dry cell to reduce any metal ions which might be formed
D)The metal pipe is protected by attaching an active metal to make the pipe the anode in an electrochemical cell
E)The metal pipe is protected by coating the pipe with a fluoropolymer to act as a source of fluoride ion (since the latter is so hard to oxidize)

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
38
Which substance does not undergo oxidation or reduction in the reaction below?
Pb + PbO2 + 2H2SO4 → 2PbSO4 + 2H2O
A)Pb
B)H2SO4
C)PbO2
D)PbSO4
E)The reaction above is not a redox reaction
Pb + PbO2 + 2H2SO4 → 2PbSO4 + 2H2O
A)Pb
B)H2SO4
C)PbO2
D)PbSO4
E)The reaction above is not a redox reaction

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
39
What is the oxidation number of chromium in K2Cr2O7?
A)+3
B)+12
C)+6
D)+7
E)+14
A)+3
B)+12
C)+6
D)+7
E)+14

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
40
What is the anode in an alkaline battery?
A)MnO2
B)KOH
C)Zn powder
D)Mn2O3
E)Pt
A)MnO2
B)KOH
C)Zn powder
D)Mn2O3
E)Pt

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
41
The standard cell potential (E°cell)of the reaction below is +0.126 V.The value of 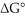 for the reaction is ________ kJ/mol. Pb (s)+ 2H+(aq)→ Pb2+ (aq)+ H2 (g)
for the reaction is ________ kJ/mol. Pb (s)+ 2H+(aq)→ Pb2+ (aq)+ H2 (g)
A)-24.3
B)+24.3
C)-12.6
D)+12.6
E)-50.8
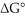 for the reaction is ________ kJ/mol. Pb (s)+ 2H+(aq)→ Pb2+ (aq)+ H2 (g)
for the reaction is ________ kJ/mol. Pb (s)+ 2H+(aq)→ Pb2+ (aq)+ H2 (g)A)-24.3
B)+24.3
C)-12.6
D)+12.6
E)-50.8

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
42
The standard cell potential (E°cell)of the reaction below is -0.55 V.The value of 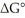 for the reaction is ________ J/mol. I2 (s)+ 2Br- (aq)→ 2I- (aq)+ Br2 (l)
for the reaction is ________ J/mol. I2 (s)+ 2Br- (aq)→ 2I- (aq)+ Br2 (l)
A)0.54
B)0.55
C)5.5 × 10-6
D)1.1 × 105
E)none of the above
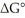 for the reaction is ________ J/mol. I2 (s)+ 2Br- (aq)→ 2I- (aq)+ Br2 (l)
for the reaction is ________ J/mol. I2 (s)+ 2Br- (aq)→ 2I- (aq)+ Br2 (l)A)0.54
B)0.55
C)5.5 × 10-6
D)1.1 × 105
E)none of the above

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
43
The less ________ the value of E°red,the lower the driving force for reduction.
A)negative
B)exothermic
C)positive
D)endothermic
E)extensive
A)negative
B)exothermic
C)positive
D)endothermic
E)extensive

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
44
In a voltaic cell,electrons flow from the ________ to the ________.
A)salt bride, anode
B)anode, salt bridge
C)cathode, anode
D)salt bridge, cathode
E)anode, cathode
A)salt bride, anode
B)anode, salt bridge
C)cathode, anode
D)salt bridge, cathode
E)anode, cathode

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
45
The half-reaction occurring at the anode in the balanced reaction shown below is ________. 3MnO4- (aq)+ 24H+ (aq)+ 5Fe (s)→ 3Mn2+ (aq)+ 5Fe3+ (aq)+ 12H2O (l)
A)MnO4- (aq)+ 8H+ (aq)+ 5e- → Mn2+ (aq)+ 4H2O (l)
B)2MnO4- (aq)+ 12H+ (aq)+ 6e- → 2Mn2+ (aq)+ 3H2O (l)
C)Fe (s)→ Fe3+ (aq)+ 3e-
D)Fe (s)→ Fe2+ (aq)+ 2e-
E)Fe2+ (aq)→ Fe3+ (aq)+ e-
A)MnO4- (aq)+ 8H+ (aq)+ 5e- → Mn2+ (aq)+ 4H2O (l)
B)2MnO4- (aq)+ 12H+ (aq)+ 6e- → 2Mn2+ (aq)+ 3H2O (l)
C)Fe (s)→ Fe3+ (aq)+ 3e-
D)Fe (s)→ Fe2+ (aq)+ 2e-
E)Fe2+ (aq)→ Fe3+ (aq)+ e-

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
46
The standard cell potential (E°cell)of the reaction below is -0.34 V.The value of 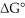 for the reaction is ________ kJ/mol. Cu (s)+ 2H+ (aq)→ Cu2+ (aq)+ H2 (g)
for the reaction is ________ kJ/mol. Cu (s)+ 2H+ (aq)→ Cu2+ (aq)+ H2 (g)
A)-0.34
B)+66
C)-130
D)+130
E)none of the above
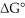 for the reaction is ________ kJ/mol. Cu (s)+ 2H+ (aq)→ Cu2+ (aq)+ H2 (g)
for the reaction is ________ kJ/mol. Cu (s)+ 2H+ (aq)→ Cu2+ (aq)+ H2 (g)A)-0.34
B)+66
C)-130
D)+130
E)none of the above

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
47
The balanced half-reaction in which sulfate ion is reduced to sulfite ion is a ________ process.
A)four-electron
B)one-electron
C)two-electron
D)three-electron
E)six-electron
A)four-electron
B)one-electron
C)two-electron
D)three-electron
E)six-electron

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
48
The balanced half-reaction in which ethanol,CH3CH2OH,is oxidized to ethanoic acid,CH3COOH. is a ________ process.
A)twelve-electron
B)three-electron
C)four-electron
D)six-electron
E)two-electron
A)twelve-electron
B)three-electron
C)four-electron
D)six-electron
E)two-electron

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
49
The standard cell potential (E°cell)for the voltaic cell based on the reaction below is ________ V. 2Cr (s)+ 3Fe2+ (aq)→ 3Fe (s)+ 2Cr3+ (aq)
A)+0.30
B)+2.80
C)+3.10
D)+0.83
E)-0.16
A)+0.30
B)+2.80
C)+3.10
D)+0.83
E)-0.16

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
50
The balanced half-reaction in which dichromate ion is reduced to chromium metal is a ________ process.
A)two-electron
B)six-electron
C)three-electron
D)four-electron
E)twelve-electron
A)two-electron
B)six-electron
C)three-electron
D)four-electron
E)twelve-electron

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
51
The reduction half reaction occurring in the standard hydrogen electrode is ________.
A)H2 (g, 1 atm)→ 2H+ (aq, 1M)+ 2e-
B)2H+ (aq)+ 2O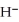 → H2O (l)
→ H2O (l)
C)O2 (g)+ 4H+ (aq)+ 4e- → 2H2O (l)
D)2H+ (aq, 1M)+ 2e- → H2 (g, 1 atm)
E)2H+ (aq, 1M)+ Cl2 (aq)→ 2HCl (aq)
A)H2 (g, 1 atm)→ 2H+ (aq, 1M)+ 2e-
B)2H+ (aq)+ 2O
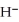 → H2O (l)
→ H2O (l)C)O2 (g)+ 4H+ (aq)+ 4e- → 2H2O (l)
D)2H+ (aq, 1M)+ 2e- → H2 (g, 1 atm)
E)2H+ (aq, 1M)+ Cl2 (aq)→ 2HCl (aq)

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
52
________ electrons appear in the following half-reaction when it is balanced. S4O62- → 2S2O32-
A)6
B)2
C)4
D)1
E)3
A)6
B)2
C)4
D)1
E)3

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
53
The relationship between the change in Gibbs free energy and the emf of an electrochemical cell is given by ________.
A)ΔG =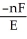
B)ΔG =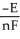
C)ΔG = -nFE
D)ΔG = -nRTF
E)ΔG =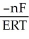
A)ΔG =
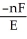
B)ΔG =
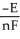
C)ΔG = -nFE
D)ΔG = -nRTF
E)ΔG =
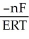

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
54
The electrode at which oxidation occurs is called the ________.
A)oxidizing agent
B)cathode
C)reducing agent
D)anode
E)voltaic cell
A)oxidizing agent
B)cathode
C)reducing agent
D)anode
E)voltaic cell

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
55
1V = ________.
A)1 amp ∙ s
B)1 J/s
C)96485 C
D)1 J/C
E)1 C/J
A)1 amp ∙ s
B)1 J/s
C)96485 C
D)1 J/C
E)1 C/J

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
56
The standard cell potential (E°cell)for the voltaic cell based on the reaction below is ________ V. Cr (s)+ 3Fe3+ (aq)→ 3Fe2+ (aq)+ Cr3+ (aq)
A)-1.45
B)+2.99
C)+1.51
D)+3.05
E)+1.57
A)-1.45
B)+2.99
C)+1.51
D)+3.05
E)+1.57

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
57
The standard cell potential (E°cell)for the voltaic cell based on the reaction below is ________ V. Sn2+ (aq)+ 2Fe3+ (aq)→ 2Fe2+ (aq)+ Sn4+ (aq)
A)+0.46
B)+0.617
C)+1.39
D)-0.46
E)+1.21
A)+0.46
B)+0.617
C)+1.39
D)-0.46
E)+1.21

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
58
The standard cell potential (E°cell)for the voltaic cell based on the reaction below is ________ V. 3Sn4+ (aq)+ 2Cr (s)→ 2Cr3+ (aq)+ 3Sn2+ (aq)
A)+1.94
B)+0.89
C)+2.53
D)-0.59
E)-1.02
A)+1.94
B)+0.89
C)+2.53
D)-0.59
E)-1.02

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
59
The balanced half-reaction in which chlorine gas is reduced to the aqueous chloride ion is a ________ process.
A)one-electron
B)two-electron
C)four-electron
D)three-electron
E)six-electron
A)one-electron
B)two-electron
C)four-electron
D)three-electron
E)six-electron

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
60
The half-reaction occurring at the cathode in the balanced reaction shown below is ________. 3MnO4- (aq)+ 24H+ (aq)+ 5Fe (s)→ 3Mn2+ (aq)+ 5Fe3+ (aq)+ 12H2O (l)
A)MnO4- (aq)+ 8H+ (aq)+ 5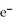 → Mn2+ (aq)+ 4H2O (l)
→ Mn2+ (aq)+ 4H2O (l)
B)2MnO4- (aq)+ 12H+ (aq)+ 6e- → 2Mn2+ (aq)+ 3H2O (l)
C)Fe (s)→ Fe3+ (aq)+ 3e-
D)Fe (s)→ Fe2+ (aq)+ 2e-
E)Fe2+ (aq)→ Fe3+ (aq)+ e-
A)MnO4- (aq)+ 8H+ (aq)+ 5
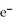 → Mn2+ (aq)+ 4H2O (l)
→ Mn2+ (aq)+ 4H2O (l)B)2MnO4- (aq)+ 12H+ (aq)+ 6e- → 2Mn2+ (aq)+ 3H2O (l)
C)Fe (s)→ Fe3+ (aq)+ 3e-
D)Fe (s)→ Fe2+ (aq)+ 2e-
E)Fe2+ (aq)→ Fe3+ (aq)+ e-

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
61
The lead-containing reactant(s)consumed during recharging of a lead-acid battery is/are ________.
A)Pb (s)only
B)PbO2 (s)only
C)PbSO4 (s)only
D)both Pb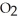 (s)and PbSO4 (s)
(s)and PbSO4 (s)
E)both Pb (s)and PbO2 (s)
A)Pb (s)only
B)PbO2 (s)only
C)PbSO4 (s)only
D)both Pb
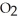 (s)and PbSO4 (s)
(s)and PbSO4 (s)E)both Pb (s)and PbO2 (s)

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
62
In the electrochemical cell using the redox reaction below,the oxidation half reaction is ________. 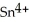 (aq)+ Fe (s)→
(aq)+ Fe (s)→ 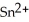 (aq)+
(aq)+ 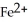 (aq)
(aq)
A)Sn4+ + 2e- → Sn2+
B)Fe → Fe2+ + 2e-
C)Sn4+ → Sn2+ + 2e-
D)Fe + 2e- → Fe2+
E)Fe +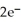 →
→ 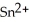
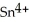 (aq)+ Fe (s)→
(aq)+ Fe (s)→ 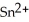 (aq)+
(aq)+ 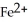 (aq)
(aq)A)Sn4+ + 2e- → Sn2+
B)Fe → Fe2+ + 2e-
C)Sn4+ → Sn2+ + 2e-
D)Fe + 2e- → Fe2+
E)Fe +
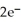 →
→ 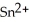

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
63
The standard cell potential ( ![<strong>The standard cell potential ( )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ] = 3.0 M and [ ] = (aq)+ Zn (s)→ (aq)+ Pb (s)</strong> A)0.51 B)0.86 C)0.40 D)0.75 E)0.63](https://storage.examlex.com/TB1194/11ea7e7c_6193_ebcf_9a0a_9358c243250a_TB1194_11.jpg) )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [
)for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ![<strong>The standard cell potential ( )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ] = 3.0 M and [ ] = (aq)+ Zn (s)→ (aq)+ Pb (s)</strong> A)0.51 B)0.86 C)0.40 D)0.75 E)0.63](https://storage.examlex.com/TB1194/11ea7e7c_6193_ebd0_9a0a_7f2b4ddee7ff_TB1194_11.jpg) ] = 3.0 M and [
] = 3.0 M and [ ![<strong>The standard cell potential ( )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ] = 3.0 M and [ ] = (aq)+ Zn (s)→ (aq)+ Pb (s)</strong> A)0.51 B)0.86 C)0.40 D)0.75 E)0.63](https://storage.examlex.com/TB1194/11ea7e7c_6193_ebd1_9a0a_ed9b581fad48_TB1194_11.jpg) ] =
] = ![<strong>The standard cell potential ( )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ] = 3.0 M and [ ] = (aq)+ Zn (s)→ (aq)+ Pb (s)</strong> A)0.51 B)0.86 C)0.40 D)0.75 E)0.63](https://storage.examlex.com/TB1194/11ea7e7c_6193_ebd2_9a0a_e5fb36e455b7_TB1194_11.jpg)
![<strong>The standard cell potential ( )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ] = 3.0 M and [ ] = (aq)+ Zn (s)→ (aq)+ Pb (s)</strong> A)0.51 B)0.86 C)0.40 D)0.75 E)0.63](https://storage.examlex.com/TB1194/11ea7e7c_6194_12e3_9a0a_4114e083f283_TB1194_11.jpg) (aq)+ Zn (s)→
(aq)+ Zn (s)→ ![<strong>The standard cell potential ( )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ] = 3.0 M and [ ] = (aq)+ Zn (s)→ (aq)+ Pb (s)</strong> A)0.51 B)0.86 C)0.40 D)0.75 E)0.63](https://storage.examlex.com/TB1194/11ea7e7c_6194_12e4_9a0a_0552831228d5_TB1194_11.jpg) (aq)+ Pb (s)
(aq)+ Pb (s)
A)0.51
B)0.86
C)0.40
D)0.75
E)0.63
![<strong>The standard cell potential ( )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ] = 3.0 M and [ ] = (aq)+ Zn (s)→ (aq)+ Pb (s)</strong> A)0.51 B)0.86 C)0.40 D)0.75 E)0.63](https://storage.examlex.com/TB1194/11ea7e7c_6193_ebcf_9a0a_9358c243250a_TB1194_11.jpg) )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [
)for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ![<strong>The standard cell potential ( )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ] = 3.0 M and [ ] = (aq)+ Zn (s)→ (aq)+ Pb (s)</strong> A)0.51 B)0.86 C)0.40 D)0.75 E)0.63](https://storage.examlex.com/TB1194/11ea7e7c_6193_ebd0_9a0a_7f2b4ddee7ff_TB1194_11.jpg) ] = 3.0 M and [
] = 3.0 M and [ ![<strong>The standard cell potential ( )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ] = 3.0 M and [ ] = (aq)+ Zn (s)→ (aq)+ Pb (s)</strong> A)0.51 B)0.86 C)0.40 D)0.75 E)0.63](https://storage.examlex.com/TB1194/11ea7e7c_6193_ebd1_9a0a_ed9b581fad48_TB1194_11.jpg) ] =
] = ![<strong>The standard cell potential ( )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ] = 3.0 M and [ ] = (aq)+ Zn (s)→ (aq)+ Pb (s)</strong> A)0.51 B)0.86 C)0.40 D)0.75 E)0.63](https://storage.examlex.com/TB1194/11ea7e7c_6193_ebd2_9a0a_e5fb36e455b7_TB1194_11.jpg)
![<strong>The standard cell potential ( )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ] = 3.0 M and [ ] = (aq)+ Zn (s)→ (aq)+ Pb (s)</strong> A)0.51 B)0.86 C)0.40 D)0.75 E)0.63](https://storage.examlex.com/TB1194/11ea7e7c_6194_12e3_9a0a_4114e083f283_TB1194_11.jpg) (aq)+ Zn (s)→
(aq)+ Zn (s)→ ![<strong>The standard cell potential ( )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ] = 3.0 M and [ ] = (aq)+ Zn (s)→ (aq)+ Pb (s)</strong> A)0.51 B)0.86 C)0.40 D)0.75 E)0.63](https://storage.examlex.com/TB1194/11ea7e7c_6194_12e4_9a0a_0552831228d5_TB1194_11.jpg) (aq)+ Pb (s)
(aq)+ Pb (s)A)0.51
B)0.86
C)0.40
D)0.75
E)0.63

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
64
What is the correct coefficient for the electrons in the following half-reaction: Ni6+ + ___e- → Ni
A)6
B)1
C)2
D)3
E)5
A)6
B)1
C)2
D)3
E)5

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
65
In the electrochemical cell using the redox reaction below,the cathode half-reaction is ________. 2 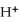 (s)+ Sn (s)→
(s)+ Sn (s)→ 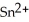 (aq)+ H2 (g)
(aq)+ H2 (g)
A)2H+ + 2e- → H2
B)Sn → Sn2+ + 2e-
C)2H+ → H2 + 2e-
D)Sn + 2e- → Sn2+
E)Sn +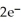 → H2
→ H2
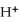 (s)+ Sn (s)→
(s)+ Sn (s)→ 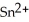 (aq)+ H2 (g)
(aq)+ H2 (g)A)2H+ + 2e- → H2
B)Sn → Sn2+ + 2e-
C)2H+ → H2 + 2e-
D)Sn + 2e- → Sn2+
E)Sn +
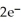 → H2
→ H2
Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
66
In the galvanic cell using the redox reaction below,the reduction half-reaction is ________. Zn (s)+ 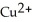 (aq)→
(aq)→ 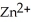 (aq)+ Cu (s)
(aq)+ Cu (s)
A)Cu2+ + 2e- → Cu
B)Zn → Zn2+ + 2e-
C)Cu2+ → Cu + 2e-
D)Zn + 2e- → Zn2+
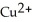 (aq)→
(aq)→ 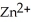 (aq)+ Cu (s)
(aq)+ Cu (s)A)Cu2+ + 2e- → Cu
B)Zn → Zn2+ + 2e-
C)Cu2+ → Cu + 2e-
D)Zn + 2e- → Zn2+

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
67
A voltaic cell is constructed with two silver-silver chloride electrodes,where the half-reaction is AgCl (s)+ 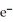 → Ag (s)+
→ Ag (s)+ 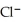 (aq)E° = +0.222 V
(aq)E° = +0.222 V
The concentrations of chloride ion in the two compartments are 0.0100 M and 1.55 M,respectively.At 25 °C,the cell emf is ________ V.
A)0.216
B)0.130
C)0.00143
D)34.4
E)0.228
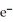 → Ag (s)+
→ Ag (s)+ 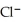 (aq)E° = +0.222 V
(aq)E° = +0.222 VThe concentrations of chloride ion in the two compartments are 0.0100 M and 1.55 M,respectively.At 25 °C,the cell emf is ________ V.
A)0.216
B)0.130
C)0.00143
D)34.4
E)0.228

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
68
Galvanized iron is iron coated with ________.
A)magnesium
B)zinc
C)chromium
D)phosphate
E)iron oxide
A)magnesium
B)zinc
C)chromium
D)phosphate
E)iron oxide

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
69
What is the oxidation number of nitrogen in the NH2OH molecule?
A)-1
B)-2
C)-3
D)0
E)+1
A)-1
B)-2
C)-3
D)0
E)+1

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
70
The standard cell potential ( 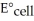 )for the reaction below is +1.10 V.The cell potential for this reaction is ________ V when the concentration of
)for the reaction below is +1.10 V.The cell potential for this reaction is ________ V when the concentration of 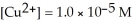 and
and 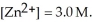 Zn (s)+
Zn (s)+ 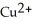 (aq)→ Cu (s)+
(aq)→ Cu (s)+ 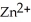 (aq)
(aq)
A)1.42
B)1.26
C)0.94
D)0.78
E)1.10
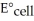 )for the reaction below is +1.10 V.The cell potential for this reaction is ________ V when the concentration of
)for the reaction below is +1.10 V.The cell potential for this reaction is ________ V when the concentration of 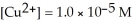 and
and 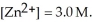 Zn (s)+
Zn (s)+ 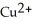 (aq)→ Cu (s)+
(aq)→ Cu (s)+ 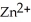 (aq)
(aq)A)1.42
B)1.26
C)0.94
D)0.78
E)1.10

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
71
Which element is reduced in the reaction below?
Fe2+ + H+ + Cr2O72- → Fe3+ + Cr3+ +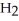 O
O
A)Cr
B)Fe
C)H
D)O
Fe2+ + H+ + Cr2O72- → Fe3+ + Cr3+ +
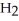 O
OA)Cr
B)Fe
C)H
D)O

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
72
The standard cell potential (E°)of a voltaic cell constructed using the cell reaction below is 0.76 V: Zn (s)+ ![<strong>The standard cell potential (E°)of a voltaic cell constructed using the cell reaction below is 0.76 V: Zn (s)+ (aq)→ (aq)+ (g) With = 1.0 atm and [ ] = 1.0 M,the cell potential is 0.53 V.At 25 °C,the concentration of in the cathode compartment is ________ M.</strong> A)1.3 × 10<sup>-4</sup> B)1.7 × 10<sup>-8</sup> C)1.1 × 10<sup>-2</sup> D)7.7 × 10<sup>3</sup> E)1.3 × 10<sup>-11</sup>](https://storage.examlex.com/TB1194/11ea7e7c_6193_9da7_9a0a_6bd39b93da47_TB1194_11.jpg) (aq)→
(aq)→ ![<strong>The standard cell potential (E°)of a voltaic cell constructed using the cell reaction below is 0.76 V: Zn (s)+ (aq)→ (aq)+ (g) With = 1.0 atm and [ ] = 1.0 M,the cell potential is 0.53 V.At 25 °C,the concentration of in the cathode compartment is ________ M.</strong> A)1.3 × 10<sup>-4</sup> B)1.7 × 10<sup>-8</sup> C)1.1 × 10<sup>-2</sup> D)7.7 × 10<sup>3</sup> E)1.3 × 10<sup>-11</sup>](https://storage.examlex.com/TB1194/11ea7e7c_6193_9da8_9a0a_375cb42bd050_TB1194_11.jpg) (aq)+
(aq)+ ![<strong>The standard cell potential (E°)of a voltaic cell constructed using the cell reaction below is 0.76 V: Zn (s)+ (aq)→ (aq)+ (g) With = 1.0 atm and [ ] = 1.0 M,the cell potential is 0.53 V.At 25 °C,the concentration of in the cathode compartment is ________ M.</strong> A)1.3 × 10<sup>-4</sup> B)1.7 × 10<sup>-8</sup> C)1.1 × 10<sup>-2</sup> D)7.7 × 10<sup>3</sup> E)1.3 × 10<sup>-11</sup>](https://storage.examlex.com/TB1194/11ea7e7c_6193_9da9_9a0a_c321e21fe3e7_TB1194_11.jpg) (g)
(g)
With![<strong>The standard cell potential (E°)of a voltaic cell constructed using the cell reaction below is 0.76 V: Zn (s)+ (aq)→ (aq)+ (g) With = 1.0 atm and [ ] = 1.0 M,the cell potential is 0.53 V.At 25 °C,the concentration of in the cathode compartment is ________ M.</strong> A)1.3 × 10<sup>-4</sup> B)1.7 × 10<sup>-8</sup> C)1.1 × 10<sup>-2</sup> D)7.7 × 10<sup>3</sup> E)1.3 × 10<sup>-11</sup>](https://storage.examlex.com/TB1194/11ea7e7c_6193_9daa_9a0a_a54fe0be2579_TB1194_11.jpg) = 1.0 atm and [
= 1.0 atm and [ ![<strong>The standard cell potential (E°)of a voltaic cell constructed using the cell reaction below is 0.76 V: Zn (s)+ (aq)→ (aq)+ (g) With = 1.0 atm and [ ] = 1.0 M,the cell potential is 0.53 V.At 25 °C,the concentration of in the cathode compartment is ________ M.</strong> A)1.3 × 10<sup>-4</sup> B)1.7 × 10<sup>-8</sup> C)1.1 × 10<sup>-2</sup> D)7.7 × 10<sup>3</sup> E)1.3 × 10<sup>-11</sup>](https://storage.examlex.com/TB1194/11ea7e7c_6193_c4bb_9a0a_c3165af66dd5_TB1194_11.jpg) ] = 1.0 M,the cell potential is 0.53 V.At 25 °C,the concentration of
] = 1.0 M,the cell potential is 0.53 V.At 25 °C,the concentration of ![<strong>The standard cell potential (E°)of a voltaic cell constructed using the cell reaction below is 0.76 V: Zn (s)+ (aq)→ (aq)+ (g) With = 1.0 atm and [ ] = 1.0 M,the cell potential is 0.53 V.At 25 °C,the concentration of in the cathode compartment is ________ M.</strong> A)1.3 × 10<sup>-4</sup> B)1.7 × 10<sup>-8</sup> C)1.1 × 10<sup>-2</sup> D)7.7 × 10<sup>3</sup> E)1.3 × 10<sup>-11</sup>](https://storage.examlex.com/TB1194/11ea7e7c_6193_c4bc_9a0a_7f84b9ad2390_TB1194_11.jpg) in the cathode compartment is ________ M.
in the cathode compartment is ________ M.
A)1.3 × 10-4
B)1.7 × 10-8
C)1.1 × 10-2
D)7.7 × 103
E)1.3 × 10-11
![<strong>The standard cell potential (E°)of a voltaic cell constructed using the cell reaction below is 0.76 V: Zn (s)+ (aq)→ (aq)+ (g) With = 1.0 atm and [ ] = 1.0 M,the cell potential is 0.53 V.At 25 °C,the concentration of in the cathode compartment is ________ M.</strong> A)1.3 × 10<sup>-4</sup> B)1.7 × 10<sup>-8</sup> C)1.1 × 10<sup>-2</sup> D)7.7 × 10<sup>3</sup> E)1.3 × 10<sup>-11</sup>](https://storage.examlex.com/TB1194/11ea7e7c_6193_9da7_9a0a_6bd39b93da47_TB1194_11.jpg) (aq)→
(aq)→ ![<strong>The standard cell potential (E°)of a voltaic cell constructed using the cell reaction below is 0.76 V: Zn (s)+ (aq)→ (aq)+ (g) With = 1.0 atm and [ ] = 1.0 M,the cell potential is 0.53 V.At 25 °C,the concentration of in the cathode compartment is ________ M.</strong> A)1.3 × 10<sup>-4</sup> B)1.7 × 10<sup>-8</sup> C)1.1 × 10<sup>-2</sup> D)7.7 × 10<sup>3</sup> E)1.3 × 10<sup>-11</sup>](https://storage.examlex.com/TB1194/11ea7e7c_6193_9da8_9a0a_375cb42bd050_TB1194_11.jpg) (aq)+
(aq)+ ![<strong>The standard cell potential (E°)of a voltaic cell constructed using the cell reaction below is 0.76 V: Zn (s)+ (aq)→ (aq)+ (g) With = 1.0 atm and [ ] = 1.0 M,the cell potential is 0.53 V.At 25 °C,the concentration of in the cathode compartment is ________ M.</strong> A)1.3 × 10<sup>-4</sup> B)1.7 × 10<sup>-8</sup> C)1.1 × 10<sup>-2</sup> D)7.7 × 10<sup>3</sup> E)1.3 × 10<sup>-11</sup>](https://storage.examlex.com/TB1194/11ea7e7c_6193_9da9_9a0a_c321e21fe3e7_TB1194_11.jpg) (g)
(g)With
![<strong>The standard cell potential (E°)of a voltaic cell constructed using the cell reaction below is 0.76 V: Zn (s)+ (aq)→ (aq)+ (g) With = 1.0 atm and [ ] = 1.0 M,the cell potential is 0.53 V.At 25 °C,the concentration of in the cathode compartment is ________ M.</strong> A)1.3 × 10<sup>-4</sup> B)1.7 × 10<sup>-8</sup> C)1.1 × 10<sup>-2</sup> D)7.7 × 10<sup>3</sup> E)1.3 × 10<sup>-11</sup>](https://storage.examlex.com/TB1194/11ea7e7c_6193_9daa_9a0a_a54fe0be2579_TB1194_11.jpg) = 1.0 atm and [
= 1.0 atm and [ ![<strong>The standard cell potential (E°)of a voltaic cell constructed using the cell reaction below is 0.76 V: Zn (s)+ (aq)→ (aq)+ (g) With = 1.0 atm and [ ] = 1.0 M,the cell potential is 0.53 V.At 25 °C,the concentration of in the cathode compartment is ________ M.</strong> A)1.3 × 10<sup>-4</sup> B)1.7 × 10<sup>-8</sup> C)1.1 × 10<sup>-2</sup> D)7.7 × 10<sup>3</sup> E)1.3 × 10<sup>-11</sup>](https://storage.examlex.com/TB1194/11ea7e7c_6193_c4bb_9a0a_c3165af66dd5_TB1194_11.jpg) ] = 1.0 M,the cell potential is 0.53 V.At 25 °C,the concentration of
] = 1.0 M,the cell potential is 0.53 V.At 25 °C,the concentration of ![<strong>The standard cell potential (E°)of a voltaic cell constructed using the cell reaction below is 0.76 V: Zn (s)+ (aq)→ (aq)+ (g) With = 1.0 atm and [ ] = 1.0 M,the cell potential is 0.53 V.At 25 °C,the concentration of in the cathode compartment is ________ M.</strong> A)1.3 × 10<sup>-4</sup> B)1.7 × 10<sup>-8</sup> C)1.1 × 10<sup>-2</sup> D)7.7 × 10<sup>3</sup> E)1.3 × 10<sup>-11</sup>](https://storage.examlex.com/TB1194/11ea7e7c_6193_c4bc_9a0a_7f84b9ad2390_TB1194_11.jpg) in the cathode compartment is ________ M.
in the cathode compartment is ________ M.A)1.3 × 10-4
B)1.7 × 10-8
C)1.1 × 10-2
D)7.7 × 103
E)1.3 × 10-11

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
73
Which element is reduced in the following reaction? 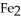
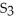 + 12HN
+ 12HN 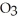 → 2Fe(N
→ 2Fe(N 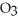
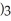 + 3S + 6N
+ 3S + 6N 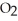 + 6H2O
+ 6H2O
A)N
B)S
C)H
D)O
E)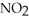
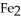
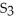 + 12HN
+ 12HN 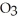 → 2Fe(N
→ 2Fe(N 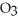
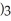 + 3S + 6N
+ 3S + 6N 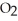 + 6H2O
+ 6H2OA)N
B)S
C)H
D)O
E)
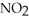

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
74
Which substance is the oxidizing agent in the reaction below?
Fe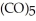 (l)+ 2HI (g)→ Fe
(l)+ 2HI (g)→ Fe 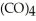
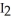 (s)+ CO (g)+
(s)+ CO (g)+ 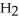 (g)
(g)
A)HI
B)Fe(CO)5
C)Fe(CO)4I2
D)CO
E)H2
Fe
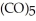 (l)+ 2HI (g)→ Fe
(l)+ 2HI (g)→ Fe 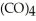
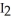 (s)+ CO (g)+
(s)+ CO (g)+ 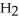 (g)
(g)A)HI
B)Fe(CO)5
C)Fe(CO)4I2
D)CO
E)H2

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
75
What is the oxidation number of bromine in the HBrO molecule?
A)+1
B)+2
C)0
D)-1
E)-2
A)+1
B)+2
C)0
D)-1
E)-2

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
76
The standard cell potential (E°cell)of the reaction below is +1.34 V.The value of 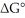 for the reaction is ________ kJ/mol. 3 Cu (s)+ 2 MnO4- (aq)+ 8H+ (aq)→ 3 Cu2+ (aq)+ 2 MnO2 (s)+ 4 H2O (l)
for the reaction is ________ kJ/mol. 3 Cu (s)+ 2 MnO4- (aq)+ 8H+ (aq)→ 3 Cu2+ (aq)+ 2 MnO2 (s)+ 4 H2O (l)
A)-24.3
B)+259
C)-259
D)+776
E)-776
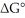 for the reaction is ________ kJ/mol. 3 Cu (s)+ 2 MnO4- (aq)+ 8H+ (aq)→ 3 Cu2+ (aq)+ 2 MnO2 (s)+ 4 H2O (l)
for the reaction is ________ kJ/mol. 3 Cu (s)+ 2 MnO4- (aq)+ 8H+ (aq)→ 3 Cu2+ (aq)+ 2 MnO2 (s)+ 4 H2O (l)A)-24.3
B)+259
C)-259
D)+776
E)-776

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
77
Corrosion of iron is retarded by ________.
A)the presence of salts
B)high pH conditions
C)low pH conditions
D)both the presence of salts and high pH conditions
E)both the presence of salts and low pH conditions
A)the presence of salts
B)high pH conditions
C)low pH conditions
D)both the presence of salts and high pH conditions
E)both the presence of salts and low pH conditions

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
78
What is the oxidation number of sulfur in the S2O32- ion?
A)+2
B)+1
C)0
D)-1
E)-2
A)+2
B)+1
C)0
D)-1
E)-2

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
79
What is the oxidation number of phosphorous in the PH3 molecule?
A)-3
B)-4
C)-5
D)+1
E)0
A)-3
B)-4
C)-5
D)+1
E)0

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
80
Which element is oxidized in the reaction below?
I- + MnO4- + H+ → I2 + MnO2 + H2O
A)I
B)Mn
C)O
D)H
I- + MnO4- + H+ → I2 + MnO2 + H2O
A)I
B)Mn
C)O
D)H

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck


