Exam 20: Electrochemistry
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
What is the oxidation number of oxygen in H2O2?
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
D
The major product of a ________ fuel cell is water.
Free
(Short Answer)
4.9/5  (41)
(41)
Correct Answer:
hydrogen
Which substance does not undergo oxidation or reduction in the reaction below?
Pb + PbO2 + 2H2SO4 → 2PbSO4 + 2H2O
Free
(Multiple Choice)
5.0/5  (32)
(32)
Correct Answer:
B
The lithium ion battery has more energy per unit mass than nickel-cadmium batteries.
(True/False)
4.9/5  (32)
(32)
The standard cell potential (E°cell)of the reaction below is -0.34 V.The value of 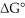 for the reaction is ________ kJ/mol. Cu (s)+ 2H+ (aq)→ Cu2+ (aq)+ H2 (g)
for the reaction is ________ kJ/mol. Cu (s)+ 2H+ (aq)→ Cu2+ (aq)+ H2 (g)
(Multiple Choice)
4.8/5  (33)
(33)
Which transformation below is an example of an oxidation in an electrochemical cell?
(Multiple Choice)
4.8/5  (44)
(44)
The standard cell potential ( ![The standard cell potential ( )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ] = 3.0 M and [ ] = (aq)+ Zn (s)→ (aq)+ Pb (s)](https://storage.examlex.com/TB1194/11ea7e7c_6193_ebcf_9a0a_9358c243250a_TB1194_11.jpg) )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [
)for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ![The standard cell potential ( )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ] = 3.0 M and [ ] = (aq)+ Zn (s)→ (aq)+ Pb (s)](https://storage.examlex.com/TB1194/11ea7e7c_6193_ebd0_9a0a_7f2b4ddee7ff_TB1194_11.jpg) ] = 3.0 M and [
] = 3.0 M and [ ![The standard cell potential ( )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ] = 3.0 M and [ ] = (aq)+ Zn (s)→ (aq)+ Pb (s)](https://storage.examlex.com/TB1194/11ea7e7c_6193_ebd1_9a0a_ed9b581fad48_TB1194_11.jpg) ] =
] = ![The standard cell potential ( )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ] = 3.0 M and [ ] = (aq)+ Zn (s)→ (aq)+ Pb (s)](https://storage.examlex.com/TB1194/11ea7e7c_6193_ebd2_9a0a_e5fb36e455b7_TB1194_11.jpg)
![The standard cell potential ( )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ] = 3.0 M and [ ] = (aq)+ Zn (s)→ (aq)+ Pb (s)](https://storage.examlex.com/TB1194/11ea7e7c_6194_12e3_9a0a_4114e083f283_TB1194_11.jpg) (aq)+ Zn (s)→
(aq)+ Zn (s)→ ![The standard cell potential ( )for the reaction below is +0.63 V.At 25 °C,the cell potential for this reaction is ________ V when [ ] = 3.0 M and [ ] = (aq)+ Zn (s)→ (aq)+ Pb (s)](https://storage.examlex.com/TB1194/11ea7e7c_6194_12e4_9a0a_0552831228d5_TB1194_11.jpg) (aq)+ Pb (s)
(aq)+ Pb (s)
(Multiple Choice)
4.7/5  (41)
(41)
Which substance is the oxidizing agent in the reaction below?
Fe 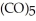 (l)+ 2HI (g)→ Fe
(l)+ 2HI (g)→ Fe 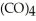
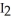 (s)+ CO (g)+
(s)+ CO (g)+ 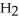 (g)
(g)
(Multiple Choice)
4.9/5  (36)
(36)
Which substance is the oxidizing agent in the following reaction?
Fe2S3 + 12HNO3 → 2Fe(NO3)3 + 3S + 6NO2 + 6H2O
(Multiple Choice)
4.8/5  (36)
(36)
A voltaic cell is constructed with two 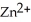 -Zn electrodes,where the half-reaction is
-Zn electrodes,where the half-reaction is 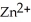 +
+ 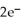 → Zn (s)E° = -0.763 V The concentrations of zinc ion in the two compartments are 4.50 M and 1.11 ×
→ Zn (s)E° = -0.763 V The concentrations of zinc ion in the two compartments are 4.50 M and 1.11 × 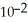 M,respectively.The cell emf is ________ V.
M,respectively.The cell emf is ________ V.
(Multiple Choice)
4.7/5  (34)
(34)
What is the oxidation number of bromine in the HBrO molecule?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the halogens in Table 20.1 is the strongest oxidizing agent?
(Multiple Choice)
4.8/5  (27)
(27)
The standard cell potential (E°cell)for the voltaic cell based on the reaction below is ________ V. 2Cr (s)+ 3Fe2+ (aq)→ 3Fe (s)+ 2Cr3+ (aq)
(Multiple Choice)
4.8/5  (36)
(36)
The standard cell potential ( 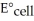 )for the reaction below is +1.10 V.The cell potential for this reaction is ________ V when the concentration of
)for the reaction below is +1.10 V.The cell potential for this reaction is ________ V when the concentration of 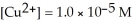 and
and 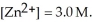 Zn (s)+
Zn (s)+ 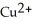 (aq)→ Cu (s)+
(aq)→ Cu (s)+ 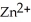 (aq)
(aq)
(Multiple Choice)
4.8/5  (34)
(34)
In the electrochemical cell using the redox reaction below,the cathode half-reaction is ________. 2 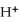 (s)+ Sn (s)→
(s)+ Sn (s)→ 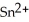 (aq)+ H2 (g)
(aq)+ H2 (g)
(Multiple Choice)
5.0/5  (36)
(36)
________ is the reducing agent in the reaction below. Cr2O72- + 6S2O32- + 14H+ → 2Cr3+ + 3S4O62- + 7H2O
(Multiple Choice)
4.9/5  (41)
(41)
Which element is reduced in the reaction below?
Fe2+ + H+ + Cr2O72- → Fe3+ + Cr3+ + 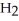 O
O
(Multiple Choice)
4.9/5  (33)
(33)
Showing 1 - 20 of 113
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)