Deck 2: The Chemical Level of Organization
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/81
Play
Full screen (f)
Deck 2: The Chemical Level of Organization
1
Which of the following subatomic particles has/have a neutral charge?
A)neutron
B)electron
C)proton
D)Both neutron and electron.
E)All of these choices.
A)neutron
B)electron
C)proton
D)Both neutron and electron.
E)All of these choices.
A
2
Which of the following subatomic particles are shared by two atoms to form covalent bonds?
1)neutron
2)electron
3)proton
A)1 only
B)2 only
C)3 only
D)2 & 3 only
E)1,2 & 3
1)neutron
2)electron
3)proton
A)1 only
B)2 only
C)3 only
D)2 & 3 only
E)1,2 & 3
B
3
Which relatively weak type of bond helps stabilize the three dimensional structure of large molecules like proteins and DNA?
A)nonpolar covalent
B)polar covalent
C)hydrogen
D)ionic
E)atomic
A)nonpolar covalent
B)polar covalent
C)hydrogen
D)ionic
E)atomic
C
4
What is the name given to a negatively charged atom?
A)superoxide
B)isotope
C)catalyst
D)anion
E)cation
A)superoxide
B)isotope
C)catalyst
D)anion
E)cation

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
5
What region of an atom contains the protons and neutrons?
A)cloud
B)nucleus
C)element
D)ring
E)shell
A)cloud
B)nucleus
C)element
D)ring
E)shell

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
6
Which term is defined as the capacity to do work?
A)metabolism
B)electrolytes
C)chemical reaction
D)concentration
E)energy
A)metabolism
B)electrolytes
C)chemical reaction
D)concentration
E)energy

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
7
An enzyme acts to
A)raise the activation energy needed to start the reaction.
B)lower the activation energy needed to start the reaction.
C)convert the activation energy into potential energy.
D)convert the activation energy into kinetic energy.
E)stop a chemical reaction.
A)raise the activation energy needed to start the reaction.
B)lower the activation energy needed to start the reaction.
C)convert the activation energy into potential energy.
D)convert the activation energy into kinetic energy.
E)stop a chemical reaction.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
8
The chemical bonds formed between the oxygen and hydrogen atoms making up a water molecule are called
A)nonpolar covalent bonds.
B)polar covalent bonds.
C)hydrogen bonds.
D)ionic bonds.
E)atomic bonds.
A)nonpolar covalent bonds.
B)polar covalent bonds.
C)hydrogen bonds.
D)ionic bonds.
E)atomic bonds.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
9
Briefly describe the octet rule.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
10
What are the four major elements found in the chemicals that comprise the human body?
A)nitrogen,oxygen,calcium,sodium
B)hydrogen,carbon,phosphorus,calcium
C)carbon,hydrogen,oxygen and nitrogen
D)oxygen,nitrogen,potassium,calcium
E)potassium,phosphorus,sodium,hydrogen
A)nitrogen,oxygen,calcium,sodium
B)hydrogen,carbon,phosphorus,calcium
C)carbon,hydrogen,oxygen and nitrogen
D)oxygen,nitrogen,potassium,calcium
E)potassium,phosphorus,sodium,hydrogen

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
11
Which type of chemical reaction will absorb more energy than it releases?
A)exergonic
B)endergonic
C)potential
D)kinetic
E)activation
A)exergonic
B)endergonic
C)potential
D)kinetic
E)activation

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
12
A chemical that can conduct electrical current when dissolved in water is called a(n)
A)isotope.
B)isomer.
C)compound.
D)electrolyte
E)valence molecule.
A)isotope.
B)isomer.
C)compound.
D)electrolyte
E)valence molecule.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
13
The nucleus of unstable _____ of an element will decay leading to emission of radiation.
A)compounds
B)cations
C)anions
D)isotopes
E)molecules
A)compounds
B)cations
C)anions
D)isotopes
E)molecules

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
14
Describe the law of conservation of energy.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
15
Describe a hydrogen bond.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
16
The number of protons in an atom is represented by an element's
A)mass number.
B)atomic number.
C)atomic mass.
D)valence number.
E)None of these choices.
A)mass number.
B)atomic number.
C)atomic mass.
D)valence number.
E)None of these choices.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
17
This refers to a weighted average of the atomic weights of all naturally occurring isotopes of an element.
A)mass number
B)atomic number
C)atomic mass
D)ionic mass
E)covalent mass
A)mass number
B)atomic number
C)atomic mass
D)ionic mass
E)covalent mass

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
18
The three types of subatomic particles that are important for understanding chemical reactions in the human body are
A)neutrons,quarks,and muons.
B)protons,neutrons,and electrons.
C)muons,positons,and neutrons.
D)electrons,quarks,and protons.
E)positons,protons,and neutrons.
A)neutrons,quarks,and muons.
B)protons,neutrons,and electrons.
C)muons,positons,and neutrons.
D)electrons,quarks,and protons.
E)positons,protons,and neutrons.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
19
Which type of chemical bond involves the sharing of valence electron pairs between two atoms?
A)covalent
B)ionic
C)hydrogen
D)atomic
E)electronic
A)covalent
B)ionic
C)hydrogen
D)atomic
E)electronic

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
20
A chemical reaction involves interactions between the _____ of two different atoms.
A)neutrons
B)protons
C)isotopes
D)valence electrons
E)ions
A)neutrons
B)protons
C)isotopes
D)valence electrons
E)ions

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
21
Specific arrangements of atoms within an organic molecule that confer characteristic chemical properties upon that molecule are called
A)hydrocarbon chains.
B)polymers.
C)carbon skeleton.
D)functional groups.
E)isomers.
A)hydrocarbon chains.
B)polymers.
C)carbon skeleton.
D)functional groups.
E)isomers.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
22
This type of lipid is the body's primary long-term energy storage molecule.
A)steroid
B)phospholipid
C)cholesterol
D)triglyceride
E)lipoprotein
A)steroid
B)phospholipid
C)cholesterol
D)triglyceride
E)lipoprotein

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
23
What is the most abundant and most important inorganic compound in the body?
A)water
B)oxygen gas
C)carbon dioxide
D)glucose
E)DNA
A)water
B)oxygen gas
C)carbon dioxide
D)glucose
E)DNA

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
24
This type of fatty acid contains more than one double bond in its hydrocarbon chain.
A)saturated
B)monounsaturated
C)polyunsaturated
D)volatile
E)short chain
A)saturated
B)monounsaturated
C)polyunsaturated
D)volatile
E)short chain

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
25
A chemical compound that helps control the pH of a solution by adding or removing hydrogen ions is a(n)
A)electrolyte.
B)salt.
C)cation.
D)colloid.
E)buffer.
A)electrolyte.
B)salt.
C)cation.
D)colloid.
E)buffer.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is a polysaccharide that serves as a storage form of energy in muscle and liver cells?
A)cellulose
B)ribose
C)lipids
D)glucose
E)glycogen
A)cellulose
B)ribose
C)lipids
D)glucose
E)glycogen

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
27
A solute that readily dissolves in water is
A)hydrophobic.
B)hydrostatic.
C)lipophilic.
D)hydrophilic.
E)hydrozone.
A)hydrophobic.
B)hydrostatic.
C)lipophilic.
D)hydrophilic.
E)hydrozone.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
28
The primary structure of a protein consists of
A)alpha helices.
B)beta-pleated sheets.
C)three dimensional folded conformation.
D)a sequence of amino acids linked by peptide bonds.
E)the overall folded conformation of the protein's subunits.
A)alpha helices.
B)beta-pleated sheets.
C)three dimensional folded conformation.
D)a sequence of amino acids linked by peptide bonds.
E)the overall folded conformation of the protein's subunits.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
29
List the six major functions of proteins.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is a proton donor?
A)acid
B)base
C)salt
D)organic compound
E)colloid
A)acid
B)base
C)salt
D)organic compound
E)colloid

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
31
Describe the structural characteristics of an amino acid.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
32
Which type of chemical reaction breaks larger reactants into smaller products?
A)synthesis
B)decomposition
C)potential
D)exchange
E)activated
A)synthesis
B)decomposition
C)potential
D)exchange
E)activated

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
33
Which type of chemical reaction combines reactants to produce larger products?
A)synthesis
B)decomposition
C)potential
D)exchange
E)activated
A)synthesis
B)decomposition
C)potential
D)exchange
E)activated

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
34
Describe the functions of water in the body.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is NOT true about phospholipids?
A)They contain a glycerol backbone.
B)The head group is polar.
C)The molecule is an important part of cell membranes.
D)The tail groups are nonpolar.
E)They are a major form of energy storage.
A)They contain a glycerol backbone.
B)The head group is polar.
C)The molecule is an important part of cell membranes.
D)The tail groups are nonpolar.
E)They are a major form of energy storage.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is a monosaccharide that is used by cells to produce energy?
A)glucose
B)sucrose
C)lactose
D)glycogen
E)maltose
A)glucose
B)sucrose
C)lactose
D)glycogen
E)maltose

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
37
In the body fluid compartments found in of the human body,the solvent is
A)glucose.
B)lipids.
C)carbon dioxide.
D)water.
E)electrolyte.
A)glucose.
B)lipids.
C)carbon dioxide.
D)water.
E)electrolyte.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
38
List three factors that increase the rate of chemical reactions.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
39
A solution with a pH value less than 7 is
A)basic.
B)neutral.
C)acidic.
D)alkaline.
E)concentrated.
A)basic.
B)neutral.
C)acidic.
D)alkaline.
E)concentrated.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
40
This lipid is used by the body as a precursor for the production of steroid hormones.
A)arachidonic acid
B)phospholipid
C)cholesterol
D)triglyceride
E)lipoprotein
A)arachidonic acid
B)phospholipid
C)cholesterol
D)triglyceride
E)lipoprotein

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is the major function of DNA?
A)catalyzes metabolic reactions
B)storage of energy transfers energy for cellular metabolism
C)transfer information carries genetic code needed for protein synthesis
D)long-term storage of information for carries inherited genetic code that controls protein synthesis
E)transports of electrolytes
A)catalyzes metabolic reactions
B)storage of energy transfers energy for cellular metabolism
C)transfer information carries genetic code needed for protein synthesis
D)long-term storage of information for carries inherited genetic code that controls protein synthesis
E)transports of electrolytes

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the labeled structures are found in DNA but not RNA?
1 A
2 B
3 C
4 E
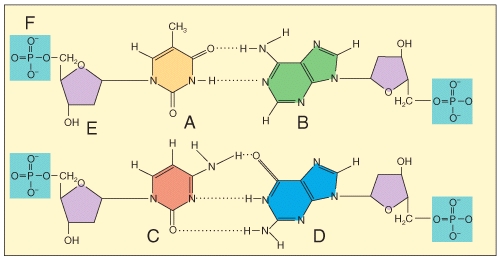
A)1 only
B)2 only
C)3 only
D)4 only
E)1 and 4
1 A
2 B
3 C
4 E

A)1 only
B)2 only
C)3 only
D)4 only
E)1 and 4

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following describes the major significance of the element nitrogen in the human body?
A)The ionized form makes body fluids acidic.
B)The ionized form is most plentiful anion in extracellular fluid.
C)The ionized form is needed for action of many enzymes.
D)It is a component of all proteins and nucleic acids.
E)The ionized form is most plentiful cation in extracellular fluid.
A)The ionized form makes body fluids acidic.
B)The ionized form is most plentiful anion in extracellular fluid.
C)The ionized form is needed for action of many enzymes.
D)It is a component of all proteins and nucleic acids.
E)The ionized form is most plentiful cation in extracellular fluid.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following carbohydrates is a polysaccharide?
A)ribose
B)lactose
C)glycogen
D)maltose
E)galactose
A)ribose
B)lactose
C)glycogen
D)maltose
E)galactose

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
45
What type of molecule is shown in the diagram? Where in a human body cell would this type of molecule be commonly found? What special chemical properties does this molecule possess that allows it to accomplish its functions?
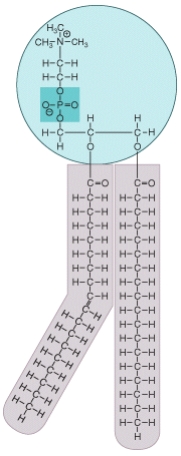


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following is a common function of RNA?
A)produces electrical impulses
B)storage of energy transfers energy for cellular metabolism
C)transfer information carries genetic code needed for protein synthesis
D)long-term storage of information for carries inherited genetic code that controls protein synthesis
E)transports of fluids
A)produces electrical impulses
B)storage of energy transfers energy for cellular metabolism
C)transfer information carries genetic code needed for protein synthesis
D)long-term storage of information for carries inherited genetic code that controls protein synthesis
E)transports of fluids

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following describes the major significance of the element carbon in the human body?
A)ionized form makes body fluids acidic
B)constituent of water
C)forms backbone of all organic molecules
D)required to harden the structure of bones and teeth
E)ionized form is the part of hemoglobin that carries oxygen
A)ionized form makes body fluids acidic
B)constituent of water
C)forms backbone of all organic molecules
D)required to harden the structure of bones and teeth
E)ionized form is the part of hemoglobin that carries oxygen

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following substances has a pH closest to 7.0?
A)lye
B)vaginal fluid
C)gastric juice
D)cerebrospinal fluid
E)milk of magnesia
A)lye
B)vaginal fluid
C)gastric juice
D)cerebrospinal fluid
E)milk of magnesia

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
49
In the diagram which particles are negatively charged?
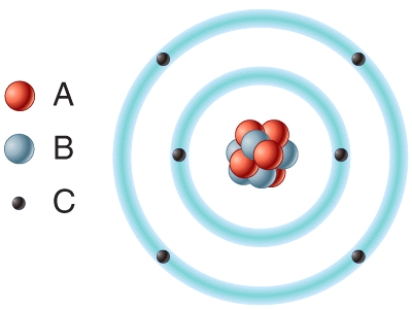
A)A
B)B
C)C
D)All of these choices.
E)None of these choices.

A)A
B)B
C)C
D)All of these choices.
E)None of these choices.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following is NOT a property of enzymes?
A)Enzymes are catalytic proteins.
B)Enzymes are highly specific.
C)Enzymes are efficient.
D)Enzymes are subject to a variety of cellular controls.
E)Enzymes are irreversibly changed by the reactions that they catalyze.
A)Enzymes are catalytic proteins.
B)Enzymes are highly specific.
C)Enzymes are efficient.
D)Enzymes are subject to a variety of cellular controls.
E)Enzymes are irreversibly changed by the reactions that they catalyze.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following carbohydrates is a disaccharide?
A)ribose
B)lactose
C)galactose
D)glycogen
E)cellulose
A)ribose
B)lactose
C)galactose
D)glycogen
E)cellulose

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
52
Which monomer is used to build RNA and DNA?
A)fatty acid
B)amino acid
C)monosaccharide
D)glycerol
E)nucleotide
A)fatty acid
B)amino acid
C)monosaccharide
D)glycerol
E)nucleotide

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following describes the major function of ATP in cells?
A)forms the building blocks for the synthesis of proteins.
B)transfers energy for cell functions
C)transfers information carries genetic code needed for protein synthesis
D)stores information for carries inherited genetic code that controls protein synthesis
E)transports fluids
A)forms the building blocks for the synthesis of proteins.
B)transfers energy for cell functions
C)transfers information carries genetic code needed for protein synthesis
D)stores information for carries inherited genetic code that controls protein synthesis
E)transports fluids

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
54
Describe what is happening at places 1,2 and 3 in the diagram.
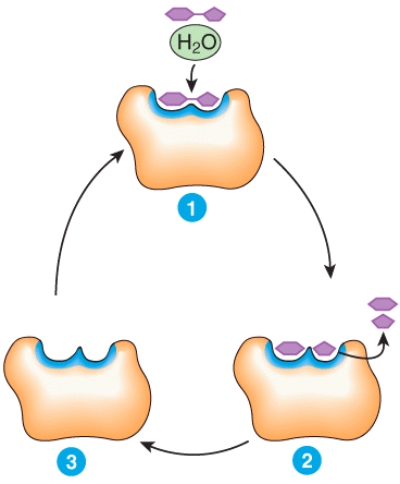


Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
55
Describe what happens to a protein's structure and function when it is denatured.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
56
In the diagram,what pH value represents an acidic Solution?
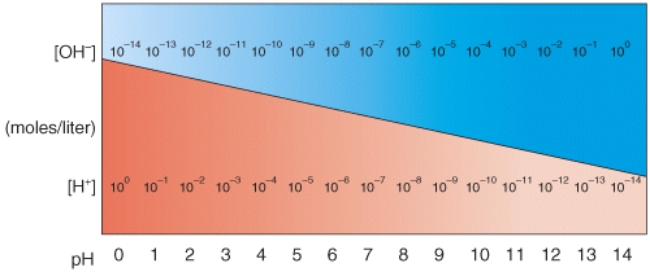
A)12
B)10
C)8
D)6
E)None of these choices.

A)12
B)10
C)8
D)6
E)None of these choices.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following describes the major significance of the element chlorine in the human body?
A)ionized form makes body fluids acidic
B)ionized form is most plentiful anion in extracellular fluid
C)forms backbone of all organic molecules
D)required for bone and tooth structure
E)ionized form is most plentiful cation in extracellular fluid
A)ionized form makes body fluids acidic
B)ionized form is most plentiful anion in extracellular fluid
C)forms backbone of all organic molecules
D)required for bone and tooth structure
E)ionized form is most plentiful cation in extracellular fluid

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
58
What is the difference between atomic mass,mass number and atomic number?

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
59
In the diagram,what would happen to the concentration of C if the concentration of A increases?
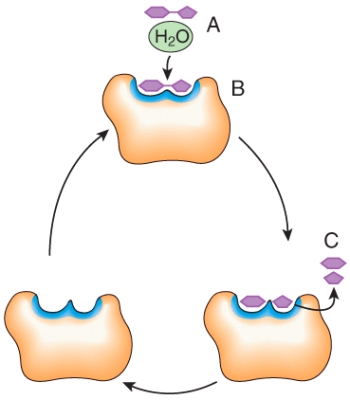
A)increases
B)decreases
C)no change

A)increases
B)decreases
C)no change

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following can lower the amount of free radicals in the body?
A)x-rays
B)ultraviolet radiation
C)oxygen
D)carbon tetrachloride
E)antioxidants
A)x-rays
B)ultraviolet radiation
C)oxygen
D)carbon tetrachloride
E)antioxidants

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
61
The energy stored in the bonds of the molecules in the foods that humans eat is
1)a form of kinetic energy.
2)a form of potential energy.
3)referred to as chemical energy.
A)1 only
B)2 only
C)3 only
D)2 and 3
E)All of these choices
1)a form of kinetic energy.
2)a form of potential energy.
3)referred to as chemical energy.
A)1 only
B)2 only
C)3 only
D)2 and 3
E)All of these choices

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
62
This type of protein is involved with shortening of muscle cells to produce movement.
A)contractile
B)structural
C)regulatory
D)catalytic
E)transport
A)contractile
B)structural
C)regulatory
D)catalytic
E)transport

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following is a general term used to refer to the sum of all the chemical reactions occurring in the body?
A)anabolism
B)catabolism
C)metabolism
D)catalysis
E)homeostasis
A)anabolism
B)catabolism
C)metabolism
D)catalysis
E)homeostasis

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
64
Define mixture and then distinguish between the three types of mixtures called Solutions ,colloids and suspensions.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
65
This type of lipid is used by the body for insulation.
A)phospholipids
B)triglycerides
C)bile salts
D)sex hormones
E)carotenes
A)phospholipids
B)triglycerides
C)bile salts
D)sex hormones
E)carotenes

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
66
A triple covalent bond is formed between atoms sharing _____ valence electrons.
A)one
B)two
C)three
D)six
E)eight
A)one
B)two
C)three
D)six
E)eight

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
67
Vitamin D is synthesized from cholesterol.What is true about Vitamin D?
A)Vitamin D is water soluble
B)Vitamin D is fat soluble
C)Vitamin D is not soluble in fat or water
D)Vitamin D is soluble in both fat and water
A)Vitamin D is water soluble
B)Vitamin D is fat soluble
C)Vitamin D is not soluble in fat or water
D)Vitamin D is soluble in both fat and water

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
68
The initial energy "investment" needed to start a chemical reaction in a cell is called the
A)energy of products.
B)energy of reactants.
C)potential energy.
D)Gibb's free energy.
E)activation energy.
A)energy of products.
B)energy of reactants.
C)potential energy.
D)Gibb's free energy.
E)activation energy.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
69
The R group of an amino acid would need to have what characteristic to be located in a cell membrane?
A)The R group would need to be polar
B)The R group would need to be nonpolar
C)The R group would need to be hydrophilic
D)The R group has to ionize
A)The R group would need to be polar
B)The R group would need to be nonpolar
C)The R group would need to be hydrophilic
D)The R group has to ionize

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
70
In a polar covalent bond,the atom that has the most electronegativity will have a
A)full negative charge (-1).
B)full positive charge (+1).
C)partial negative charge.
D)partial positive charge.
E)neutral charge.
A)full negative charge (-1).
B)full positive charge (+1).
C)partial negative charge.
D)partial positive charge.
E)neutral charge.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
71
A molecule with an unpaired electron in the outermost shell is called a(n)
A)compound.
B)free radical.
C)colloid.
D)molecule.
A)compound.
B)free radical.
C)colloid.
D)molecule.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
72
The characteristics listed below represent which element in the periodic table?
1)7 valence electrons
2)easily gains an electron
3)most likely to form an anion
4)high electronegativity
A)sodium
B)carbon
C)chlorine
D)nitrogen
E)oxygen
1)7 valence electrons
2)easily gains an electron
3)most likely to form an anion
4)high electronegativity
A)sodium
B)carbon
C)chlorine
D)nitrogen
E)oxygen

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
73
This type of protein protects against pathogens.
A)contractile
B)immunological
C)regulatory
D)catalytic
E)transport
A)contractile
B)immunological
C)regulatory
D)catalytic
E)transport

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
74
Choose the appropriate atomic number associated with the element.
Carbon's atomic mass (P = 6,N = 6)is [dropdown 1a].Carbon has [dropdown 2b] valence electrons.Carbon can form up to [dropdown 3c] covalent bonds.Carbon 14 is a radioactive isotope and contains [dropdown 4d] neutrons.
Dropdown choices
2
4
6
7
8
12
14
Carbon's atomic mass (P = 6,N = 6)is [dropdown 1a].Carbon has [dropdown 2b] valence electrons.Carbon can form up to [dropdown 3c] covalent bonds.Carbon 14 is a radioactive isotope and contains [dropdown 4d] neutrons.
Dropdown choices
2
4
6
7
8
12
14

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
75
What functional groups are present on the molecule in the diagram.Choose all that apply.
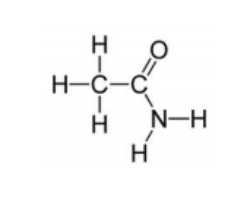
A)Hydroxyl
B)Carbonyl
C)Carboxyl
D)Ester
E)Amino

A)Hydroxyl
B)Carbonyl
C)Carboxyl
D)Ester
E)Amino

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
76
AB + CD → AD + BC is a general example of a(n)_____ reaction.
A)decomposition
B)synthesis
C)exchange
D)reversible
E)catalyzed
A)decomposition
B)synthesis
C)exchange
D)reversible
E)catalyzed

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
77
What organic compound is lacking the human digestive system that makes it unable to digest cellulose?

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
78
If there is 24% Adenine present in a DNA helix,how much thymine would be present?
A)12% thymine
B)24% thymine
C)26% thymine
D)52% thymine
E)75% thymine
A)12% thymine
B)24% thymine
C)26% thymine
D)52% thymine
E)75% thymine

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
79
In laboratory,Sudan IV is used to test for the presence of hydrophobic substances in food.Which organic molecule would exhibit a positive reaction with Sudan IV?
A)Lipids
B)Nucleic Acids
C)Carbohydrates
D)Globular proteins
A)Lipids
B)Nucleic Acids
C)Carbohydrates
D)Globular proteins

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
80
All of the following are characteristics of anabolism EXCEPT:
A)It involves synthesizing new biomolecules.
B)It primarily involves endergonic reactions.
C)It releases large amounts of energy.
D)An example of anabolism is linking amino acids together to form proteins.
E)An example is the formation of two ammonia molecules from one nitrogen molecule and three hydrogen molecules.
A)It involves synthesizing new biomolecules.
B)It primarily involves endergonic reactions.
C)It releases large amounts of energy.
D)An example of anabolism is linking amino acids together to form proteins.
E)An example is the formation of two ammonia molecules from one nitrogen molecule and three hydrogen molecules.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck


